Sulphides: Arsenopyrite
 Diagnostic card.
Diagnostic card.
In the photo: a prismatic crystal in quartz (Alpe Pickles, Valseena). Below: a druse with short-prismatic crystals of arsenopyrite (Panashkeira, Portugal)
Fe As S
Singonia monoclinic
Hardness 5,5-6
Specific weight 5,9-6,2
Cleavage is good
Crack irregular
Color from white to gray
Color in powder black
Glitter metal
Arsenopyrite (arsenic pyrite), - iron sulphosenid. The gloss is metallic, opaque. The color is tin-white with yellow turgidity. The line is black. The fracture is uneven. It's fragile. Cleavage is perfect. The crystals (of the monoclinic syngony) are columnar, needle-shaped with a sharp longitudinal shading. Solid masses are granular to drainage. The main ore is arsenic. Places of distribution: Germany (Harz, Ore Mountains, Saxony), Sweden. England.
The arsenopyrite composition rarely corresponds to the ideal formula. More often it contains a small amount of other metals, including gold, silver, cobalt. The latter can also be partly replaced by iron; In this case a kind of arsenopyrite is formed, called danaite. Crystallized in monoclinic syngony, although its crystals have a distinct pseudo-morphic appearance, and can be prismatic. The edges are curved and shaded. Frequent doubles, which have the form of a cross or a star, and are very effective. Massive accumulations of dense or granular composition are also common. Opaque. Color whitish to steel gray. The glitter is typically metallic. The mineral is hard and very heavy, brittle, easily disintegrating along cleavage planes. In powder - black.




Chalcopyrite, epitaxially accreting to the heads of an arsenopyrite crystal. Arsenopyrite in quartz.
Yaogangxiang Mine, Yizhang, Hunan, China (Hunan, China). (2011).
Diagnostic signs.
When struck with a hammer, arsenopyrite sparks, like pyrite, with the release of garlic smell, indicating the presence of arsenic. Pulverized, when heated in a test tube, first forms flakes covered with pollen of arsenic sulphide of pinkish-yellow color, which precipitates from vapors on the walls of the tube. With continued heating, the powder becomes gray-black and converted to metallic arsenic.
It melts in a reducing flame, producing a garlic smell, an arsenic mirror is formed in a closed tube. Behavior in acids. Soluble in HNO3 with the precipitation of sulfur and arsenic oxide. Chemical composition. Iron (Fe) 34.3%, arsenic (As) 46.0%, sulfur (S) 19.7%. Cobalt is often found in impurities. Often, arsenopyrite occurs in association with native gold. Well-formed crystals in druses; Usually rhombic, short-columned, pinnate; Hatching on the faces parallel to the c axis is characteristic.
Origin.
Arsenopyrite is a common mineral; It occurs in different types of rocks. Basically hydrothermal. Crystallizes one of the first. Occurs in pegmatites, as well as in gneisses and other metamorphic rocks. In ore deposits, arsenopyrite is often found in gold-quartz veins and is accompanied by tin minerals.
Place of Birth.
In Italy it is rare, found in many places. It occurs in gold-ore quartz veins, which are being developed in the Monte Rosa zone, where in its time there were many gold mines. There were beautiful crystals of arsenopyrite; Good samples are also present in the pegmatites of Peonia (Lecce province) and the ancient Kalchercinicaal-Lago mine (Trento province). In the recent past arsenopyrite in the form of dense masses was so widespread in Baku-Lochchi (near the town of Villaputso, Cagliari region) that it was developed there in an industrial way.
Application.
Arsenopyrite is the main ore mineral from which arsenic is extracted. It is also an excellent mineral raw material for the production of silver, gold, cobalt and other metals, which are by-products of the main production.

Arsenopyrite. A druse of prismatic crystals with calcite spherulites. Freiberg, Saxony, Germany. Photo: © А.А. Evseev.


Arsenopyrite vein. Trifonovskaya Sh., Kochkarskoye deposit (Au), Plast, Ural, Russia. Arsenic. Photo: © А.А. Evseev.

Arsenic. Belorechenskoye deposit, Sever. Caucasus, Russia. ~ 10x7 cm. Photo: © А.А. Yevseyev.

Arsenic. Bau, about. Kalimantan, Malaysia. Photo: © А.А. Evseev.
 Arsenopyrite is a mixture of arsenic and iron sulphide and belongs to the same type as pyrite or iron sulphide (also for a deceptive similarity to silver called "silver fools"). But if pyrite can be considered relatively harmless, then this can not be said of arsenopyrite containing heavy additions of arsenic.
Arsenopyrite is a mixture of arsenic and iron sulphide and belongs to the same type as pyrite or iron sulphide (also for a deceptive similarity to silver called "silver fools"). But if pyrite can be considered relatively harmless, then this can not be said of arsenopyrite containing heavy additions of arsenic.
A characteristic feature of the mineral is a strong "garlic" smell (so arsenic smells in general and carcinogenic vapors from arsenopyrite in particular) arising from mechanical exposure or heating. For example, if you strike a natural ingot with a hammer, sparks are cut and a garlic smell appears for a short while, then dangerous arsenic pyrite is right in front of you. In a similarity to silver and other silver metals (platinum, etc.), there is a danger of a person reflexively wishing to hold such "wealth" in hands or at least touch it. And if you do not thoroughly wash your hands and go to prepare food, and then use it, the consequences will be the most serious.
Toxic effect can produce dust arsenopyrite minerals (accumulates in the lungs and bronchi). The arsenic contained in them causes the following symptoms of poisoning: abdominal pain, upset of the stool, difficulty swallowing, vomiting, lowering blood pressure, dehydration. Stupor (inhibition), coma (unconsciousness), convulsions (contractures) and death (brain) are also possible. With minor amounts of arsenic, a slight poisoning (fever, insomnia, loss of appetite, liver and kidney damage, etc.) may appear.
Arsenic-containing compounds are widely used in agriculture, various industries, as well as in medicine. Arsenic itself is low in toxicity, and its compounds are very poisonous. Compounds of trivalent arsenic are more toxic than pentavalent compounds. However, pentavalent arsenic compounds can be reduced to trivalent arsenic in the body. The toxic dose of arsenic preparations for oral administration (in terms of pure arsenic) is 0.01 g, lethal - 0.1-0.6 g.
Arsenic compounds penetrate the body through the respiratory tract and digestive apparatus. Accumulate in the body most of all in the bones, kidneys, mucous membranes of the intestines, liver, spleen and, especially, in hair, nails and skin. Arsenic is secreted slowly, mainly by kidneys and intestines, and also by sweat glands, with milk from nursing women. Cyclic organic and inorganic arsenic compounds are formed in an organism such as arsenites, which are characterized by the strength of chemical bonds and high toxicity.
In acute poisoning with arsenic preparations, the function of the central nervous system and the circulatory system is disrupted first of all (weakening of cardiac activity, paralysis of capillaries). Dehydration of the body due to persistent vomiting and diarrhea leads to blood thickening, hemolysis of erythrocytes, acidosis. Liver and kidneys are affected, resulting in acute liver-kidney failure. Depending on the nature of the clinical picture, there are two forms of acute poisoning with inorganic arsenic preparations - gastrointestinal and paralytic.
With gastrointestinal form after 30 minutes - 2 hours or more after taking arsenic, there is a metallic taste in the mouth, scratching in the mouth and throat, pain and burning along the esophagus (behind the sternum), persistent repeated vomiting, severe abdominal pain. Because of rapid dehydration, thirst, loss of tissue turgor, voice becomes hoarse or silent, there is pain and cramps in the calf muscles. There is an increase and soreness of the liver, hemoglobinuria, oliguria or anuria, cold extremities. In severe cases, there are cyanosis (blue lips, nails, skin) due to inadequate breathing, lowering blood pressure with frequent, poor pulse filling, general convulsive reaction. Lethal outcome occurs 1-2 days after taking the poison.
The paralytic form is observed when large amounts of inorganic arsenic compounds are ingested. In this case, for several hours after the poisoning develop weakness, adynamia, a sense of fear, deafness. There are jerking in the calf muscles, convulsions, loss of consciousness, collapse, coma and death from stopping breathing. The digestive apparatus is not affected or changes insignificantly. When arsenic hits the stomach - immediate rinsing with warm water and a suspension of activated carbon.
After the red cinnabar (mercury sulphide, salt of the liquid under the Earth's metal conditions), arsenic (arsen, As) and its compounds (arsenates) are the main weapons of Europe's poisoners (Borgia). The compounds of cinnabar was poisoned by Napoleon on St. Helena (Europe). In Rome, the Locust poisons were famous; In Venice they kept poisoners (gondolas). Symptoms of arsenic poisoning are metallic taste in the mouth, vomiting, severe abdominal pain, convulsions, paralysis, death. In small doses arsenic is needed for the human body: it prevents the loss of phosphorus.
The human body contains about 15 mg of arsenic. In the human body, arsenic compounds come with drinking and mineral water, grape wines and juices, seafood, medical preparations (pharmacology), pesticides and herbicides. About 80% of arsenic is absorbed in the gastrointestinal tract, 10% through the lungs and about 1% through the skin.
More than 90% of inorganic arsenic compounds are soluble and absorbed. Then arsenic moves to the liver, where it is methylated. Accumulates in the lungs, liver, skin and small intestine. It is deposited mainly in the reticuloendothelial system, as a result of the connection of arsenite with SH-groups of proteins (aggressive). After 24 hours, 30% of arsenic is excreted in the urine and about 4% - with feces. Minor amounts are removed with sweat, with dropped hair, peeling skin and bile.
Arsenic influences oxidative processes in the mitochondria, participates in the nucleic acid exchange, i.e. Has a direct relationship to the synthesis of protein, and is necessary for the synthesis of hemoglobin, although it is not included in its composition (catalyst). Signs of arsenic deficiency: in humans - dermatitis, anemia; In animals - decreased growth and abnormal reproduction, characterized by high perinatal mortality. Other symptoms: decreased serum triglyceride concentration.
Excess arsenic in the diet causes abnormal fecundity in the animals ("rabbit effect"), which is characterized by a significant increase in sexual activity and fertility. Causes of excess arsenic: excessive intake (constant contact with arsenic, environmental pollution, smoking, grape wine and juice abuse - the "effect of Greece", long-term administration of drugs), a violation of the regulation of arsenic exchange; Accumulation in the body of arsenic with insufficiency of selenium.
The main manifestations of excess arsenic: irritability, headaches, impaired liver function, the development of fatty hepatosis; Skin allergic reactions, eczema, dermatitis, itching, ulcers, depigmentation of the skin, palmar-plantar hyperkeratosis; conjunctivitis; Defeat of the respiratory system (fibrosis, allergosis, breakthrough of the nasal septum, tumors); Vascular lesions (primarily - lower limbs - endoangiitis), nephropathy, an increased risk of developing neoplasms of the skin, liver, lungs. Remote consequences of intoxication: decrease of hearing acuity in children, nervous system damage (encephalopathy, speech disorders, coordination of movements, convulsions, psychoses, polyneuritis with pain syndrome), violation of muscle trophism, immunodeficiency.
In acute arsenic poisoning, abdominal pain, vomiting, diarrhea, and depression of the central nervous system are observed; Develop: intravascular hemolysis, acute renal, hepatic insufficiency, cardiogenic shock. The similarity of the symptoms of arsenic poisoning with the symptoms of cholera allowed the use of arsenic compounds (arsenic trioxide) as a deadly poison. In areas where there is an excess of arsenic, it accumulates in the thyroid gland in humans and causes endemic goiter. Arsenic in small doses is carcinogenic.
Arsenious mineral water is used in the treatment of anemia and gastrointestinal diseases. It is part of the mummy, a mineral-organic substance. It is used in the treatment of sleeping sickness. Arsenic is necessary: with inflammatory processes caused by protozoal and microbial damage, with allergies, anemia, to increase appetite. In experiments, it was possible to reduce the incidence of cancer with the help of selected doses of arsenic (after surgery to remove lymph nodes).
Food sources of arsenic: fish, shellfish, shrimp, krill, lobsters, lobsters, laminaria (sea kale), wild rice, corn and cereal products, lentils, carrots, grapes, strawberries, raisins. Significant amounts of arsenic are found in fish oil and marine fish (up to 10 mg / kg), wines (up to 1 mg / l and more). In arsenic water, less than 10 μg / l, in some regions of the world (India, Bangladesh, Taiwan, Mexico), the content of the element reaches 1 mg / l, which is the cause of an overdose of arsenic and requires the development of a sanatorium system (kimberlite).
ADR 6.1
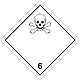
Toxic substances (poison)
Risk of poisoning by inhalation, in contact with skin or if swallowed. Dangerous to aquatic environment or sewer system
Use a mask for emergency leaving the vehicle
White diamond, ADR number, black skull and crossbones
ADR 2.3
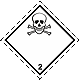
Toxic gases . Skull and crossbones
Danger of poisoning. Can be under pressure. May cause burns and / or frostbite. Capacities can explode when heated (highly dangerous - instantaneous gas spreading around the neighborhood)
Use the mask for emergency leaving the vehicle. Use shelter. Avoid low areas of the surface (pits, lowlands, trenches)
White diamond, ADR number, black skull and crossbones
| The name of a cargo that is particularly dangerous for transportation | room
UN |
Class
ADR |
| IRON (II) ARSENATE (arsenopyrite) | 1608 | 6.1. |
| IRON (III) Arsenate (arsenopyrite) | 1606 | 6.1. |
| IRON (III) Arsenite (arsenopyrite) | 1607 | 6.1. |
| AMMONIA Arsenate | 1546 | 6.1. |
| Arsenic anhydride, see ARSENA Trioksid | 1561 | 6.1. |
| ARSEN | 1558 | 6.1. |
| ARSEN dust | 1562 | 6.1. |
| Arsenic hydrogen see Arsin | 2188 | 2 |
| Arseno-soda solution | 1556 | 6.1. |
| ARSENA Bromide | 1555 | 6.1. |
| ARSENA Pentaoxide | 1559 | 6.1. |
| ARSENA CONNECTION LIQUID, NZK Inorganic, including: Arsenates, nzs, Arsenite, nzs, but ARSENA sulphides, nz | 1556 | 6.1. |
| ARSENA CONNECTION SOLID, NZK Inorganic, including: Arsenates, nzs, Arsenite, nzs, but ARSENA sulphides, nz | 1557 | 6.1. |
| ARSENA Trioside | 1561 | 6.1. |
| ARSENA Trichloride | 1560 | 6.1. |
Poisonous and radioactive dangerous stones and minerals
** - poisonous stones and minerals (mandatory check in the chemical laboratory + explicit indication of toxicity)
** - radioactive stones and minerals (mandatory check on the standard dosimeter + ban on open sales in case of radioactivity exceeding 24 milli / g / h + additional measures of population protection)
Catalog of minerals and semi-precious stones of the world by groups
** - poisonous stones and minerals
** - radioactive stones and minerals


Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.