Sulphides: Sphalerite (zinc blende)
 Diagnostic card.
Diagnostic card.
Two samples of sphalerite included in the carbonate rock
Zn S (zinc sulphide)
Cubic amount of cubic zirconia
Hardness 3,5-4
Specific weight 3,9-4,2
Cleavage is perfect
Color is colorless, diverse
Color in powder whitish, brownish
Shine from resin to semimetallic
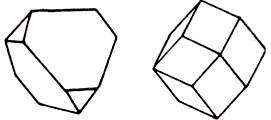
Sphalerite (zinc blende), - zinc sulphide. The gloss is diamond, less metal-like, dull, in the fracture is oily. Color honey-yellow, brown to black (depends on the iron content). Scabbard blend - fusion of sphalerite with wurtzite (hexagonal modification of ZnS). The line is yellow to brown. Fracture stepped. It's fragile. Cleavage is perfect.
It occurs mostly in hydrothermal, as well as in sedimentary deposits. Crystals (cubic syngony) are usually distorted. Polysynthetic twinning causes shading on the faces. Ordinarily, it forms fine-grained aggregates with well-defined cleavage planes. The most important zinc ore. Places of distribution: Germany, Sweden, Spain, CIS, USA.
 In addition to zinc and sulfur, sphalerite (its other name - zinc blende) can contain many other elements. Among them - iron (the limit of 20% - a kind of marmatizes) manganese, cadmium, and sometimes also indium and gallium (Ga). It crystallizes in cubic syngony, mainly in the form of tetrahedra and rhombododecahedrons. Sometimes these are complex shapes, often with streaky and curved facets. There are also solid masses - granular, fibrous and kidney-shaped. The color of sphalerite is very variable, from colorless to black, through brown tones - to yellow, pink, green. The color of the powder varies from white to brownish; Shine from resin to diamond, up to almost metallic in marmatite. The mineral can be transparent, translucent, and also opaque, if it contains a lot of iron.
In addition to zinc and sulfur, sphalerite (its other name - zinc blende) can contain many other elements. Among them - iron (the limit of 20% - a kind of marmatizes) manganese, cadmium, and sometimes also indium and gallium (Ga). It crystallizes in cubic syngony, mainly in the form of tetrahedra and rhombododecahedrons. Sometimes these are complex shapes, often with streaky and curved facets. There are also solid masses - granular, fibrous and kidney-shaped. The color of sphalerite is very variable, from colorless to black, through brown tones - to yellow, pink, green. The color of the powder varies from white to brownish; Shine from resin to diamond, up to almost metallic in marmatite. The mineral can be transparent, translucent, and also opaque, if it contains a lot of iron.
Chemical composition-content (in%): Zn-67.1; S, 32.9; Of the impurities, iron (.momatite, more than 25% Fe-kristofchg) is the most characteristic, cadmium (pribramite), gallium, manganese, mercury, etc. are more rare. Hexatetrahedral type of symmetry. Cleavage is the perfect rhombododecahedron (110). Occurs in crystals of tetrahedral, less often rhombo-decahedral habit. The main simple forms are: (111), (101), (100), (110), (113), (112), (122) and others. Hatching, steps and growth spirals are often observed on the faces. Often, twins in (111), including polysynthetic twins, are noticeable by parallel shading on cleavage planes. Solid sphalerite masses are common. Kidney-shaped cryptocrystalline aggregates of concentric-zonal structure, stalactites, are rarely noted. Epitaxial intergrowths with chalcopyrite and para-morphosis by wurtzite are known.
Diagnostic signs.
There are varieties of very hard, heavy with perfect cleavage, with a fracture of the type of the shell. Some species exhibit triboluminescence (luminescence under mechanical influences). There is also fluorescence of pink color in ultraviolet rays.
Origin.
Sphalerite is a very common mineral. It is formed in a variety of geological conditions. It is found, for example, in hydrothermal deposits, both high- and low-temperature, where it is often associated with galena, chalcopyrite, marcasite, pyrite and veined mass from quartz, barite and fluorite. It is also found in limestones affected by hydrothermal processes.

Deposits and applications.
Large deposits are found in Germany, Romania, Spain, France, Sweden, England, Scotland, Japan, Australia, Mexico, USA. In Italy, the most significant deposit (primarily from the point of view of well-educated crystals) is Bottino, in the vicinity of Serravesza, in the Padua Alps. From here came excellent specimens of sphalerite in association with chalcopyrite, meninginite, jamsonite, galena, etc. Crystals of unsurpassed beauty, perfect form and color are found in the ghosts in the marbles of Carrara and in the dolomites of Valle di Binn in Valais. Sphalerite is the most important ore for obtaining zinc, as well as rare dispersed elements: cadmium, gallium and indium.





The brilliance is diamond. Amber-yellow sphalerite is also called honey blende, orange-red - ruby blende. Polishing takes with difficulty - prevents perfect cleavage in three directions. Varieties suitable for cutting are found in Spain (Santander) and in Mexico (Chivor). Confound sphalerite can be with many yellow jewelry stones, and colorless - even with a diamond.

Sphalerite, quartz. Dalnegorsk, Primorye, Russia. Photo: © А.А. Evseev.

Sphalerite, Tl-sod. Spherolitic, kidney-shaped, rhythmic aggregate, with galena.
Reibl, Friuli-Venezia Giulia, Italy (EU). Photo: © А.А. Evseev.

Sphalerite (wurtzite). Beregovo, Transcarpathia, Ukraine (CIS). Photo: © А.А. Evseev.


Sphalerite (Kleofan). Split puncture. Picos de Europa, Santander, Spain (EU). Photo: © А.А. Evseev.
Biological role. Zinc is necessary for the functioning of DNA and RNA polymerases that control the processes of transmission of hereditary information and protein biosynthesis and reparative processes in the body; As well as the enzyme of the key reaction of heme biosynthesis, which is included in the structure of hemoglobin, cytochrome respiratory chains of mitochondria, cytochrome P-450, catalase and myeloperoxidase. Zinc is part of the key antioxidant enzyme - (Zn, Cu) superoxide dismutase - and induces the biosynthesis of the protective proteins of the metallothionein cell, - zinc is an antioxidant of the reparative effect.
Signs of insufficiency. Loss of taste and smell; Fragility, exfoliation of nails, the formation of white spots on them; Acne; Delay in puberty; fatigue; Growth retardation, hair loss; Increased cholesterol; Weakening of the acuity of night vision; Infertility, impotence, dysfunction of the prostate gland; Increased susceptibility to infections; Weakening memory; Predisposition to diabetes; Skin lesions and slow healing of wounds. The content of zinc is reduced for chronic liver and kidney diseases, in the presence of tumors, burns and myocardial infarction.
The main manifestations of excess zinc: violations of the immune system, autoimmune reactions; Violations of the skin, hair, nails; Painful sensitivity of the stomach, nausea; Reduction in iron, copper, cadmium in the body; Weakening of the functions of the prostate; Weakening of the pancreas; Weakening of the liver. The content of zinc increases with anemia, leukemia, atherosclerosis, hypertension, hyperthyroidism, overwork, stress. Nails - without white spots.
Zinc is necessary: for the prevention of acne, with prostatitis, impotence, hyperlipidemia. The daily requirement of the male body in zinc is more than that of women, and is 15-25 mg. Zinc absorption occurs in the small intestine. The main path of excretion is the gastrointestinal tract. The amount of zinc, secreted into the gut, varies depending on its consumption. About 400-600 mcg is excreted daily in urine. Zinc is lost with sperm and menstrual secret. The states of catabolism (burns, surgeries, injuries, starvation) lead to a clinically significant increase in the loss of zinc in the urine. Surface loss by desquamation (exfoliation) of the skin, with hair growth and with sweat is up to 1 mg / day. In the initial period of puberty, when the sexual organs are formed, boys need an increased amount of zinc. Girls need zinc during this period in less quantity - for overall growth and development. In adult men, against a background of zinc deficiency, a decrease in the fertilizing capacity of spermatozoa can occur.
Zinc is necessary for protein synthesis, including collagen, therefore it has wound and ulcer healing effects, participates in the processes of taste perception and smell, is necessary for the functioning of the central nervous system, including for memory processes. Zinc is vitally important for the functioning of the thymus and the normal state of the body's immune system. Being, in addition, a component of the retinol-carrying protein, zinc together with vitamin A (and vitamin C) prevents the emergence of immunodeficiencies, stimulating the synthesis of antibodies and providing antiviral action. Zinc regulates the level of metabolite testosterone - dihydrotestosterone, the excess of which causes prostate hyperplasia. Zinc is an essential factor for the female body, as it enters the structure of receptors for estrogen, thus regulating all estrogen-dependent processes.
Zinc is required to maintain a normal concentration of vitamin E in the blood, it increases the absorption of vitamin E. Zinc is indispensable for normal growth and development, so the need for zinc increases during pregnancy. This need is small: 100 mg for the entire period of pregnancy. It is especially important to ensure the need for zinc fetus in the first 3 months of pregnancy. The development of the placenta during this period requires large mineral reserves. A pregnant woman complains of changes in perception of smell and taste. This is the result of a lack of zinc in the taste buds of the tongue or in the receptors of the nasal cavity. Zinc deficiency in pregnant women can lead to atonic bleeding, premature birth, prolonged birth process.
There is evidence that gallium (a component of sphalerite) enters the shells of red blood cells and prevents the formation of thrombi. Basically, gallium enters the body with food and is contained in tissues in small amounts (0.01-0.06 μg / g). There is evidence of the presence of gallium in the glands of internal secretion, in particular, in the pituitary gland. "Depot" gallium - bone tissue and liver. The main manifestations of excess gallium: with poisoning, there is damage to the nervous system, which is accompanied by morphological changes in the liver and kidneys. There are significant fluctuations in the content of potassium and sodium in the serum, damage to the mucous membranes of the gastrointestinal tract (caustic, corrodes tissues).
The use of gallium arsenide (the production of semiconductors) since the early 80's of the XX century. Has led to an increased risk of intoxication by this element not only of workers in the electronics industry, but also of the population. The main "target" for arsenide (poisonous compounds of arsenic - such as sulphide) gallium in the body is the immune system. This element is able to disrupt the formation of gels (silica - opals in the body), due to increased excretion of aminolevulinic acid and porphyrins.
Gallium is necessary: in medicine, gallium nitrate is used in the treatment of hypercalcemia in cancer patients, where the effect of action is achieved by suppressing the activity of osteoclasts. Gallium radioisotopes are used in the diagnosis and treatment of neoplastic diseases. Gallium (itself) is relatively little toxic. The possibility of using gallium for the treatment of chronic infectious diseases of the lungs (including, possibly, tuberculosis) and cystic fibrosis (cystic fibrosis) is being studied. Food sources of gallium: wheat groats (especially - semolina), honey, many kinds of fungi (including ceps).
ADR 4.1

Highly flammable solids , self-reactive substances and solid desensitized explosives
Risk of fire. Flammable or combustible substances can ignite from sparks or flames. May contain self-reactive substances capable of exothermic decomposition in the case of heating, contact with other substances (such as: acids, heavy metal compounds or amines), friction or impact.
This can lead to the emission of harmful or flammable gases or vapor or spontaneous combustion. Containers can explode when heated (over-dangerous - practically do not burn).
Risk of explosion of desensitized explosives after loss of desensitizer
Seven vertical red stripes on a white background, equal in number, ADR number, black flame
ADR 4.3


Substances that emit flammable gases in contact with water
Risk of fire and explosion if exposed to water.
The cargo, which crumbled, must be covered and kept dry
Blue and blue diamond, ADR number, black or white flame
ADR 5.1
Substances that are oxidized
Risk of violent reaction, ignition or explosion if exposed to flammable or flammable substances
Do not allow the formation of a mixture of cargo with flammable or combustible substances (eg sawdust)
Yellow diamond, ADR number, black flame above the circle
ADR 6.1
Toxic substances (poison)
Risk of poisoning by inhalation, in contact with skin or if swallowed. Dangerous to aquatic environment or sewer system
Use a mask for emergency leaving the vehicle
White diamond, ADR number, black skull and crossbones
ADR 8
Corrosive (corrosive) substances
Risk of burns from skin corrosion. They can react violently with each other (components), with water and other substances. The substance that spilled / crumbled can emit a corrosive vapor.
Dangerous to aquatic environment or sewer system
White upper half of diamond, black - lower, equal, ADR number, test tubes, hands
ADR 9
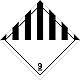
Other dangerous substances and articles
Risk of burns. Risk of fire. Risk of explosion.
Dangerous to aquatic environment or sewer system
Seven vertical black stripes on white background - top, white - lower half of diamond, ADR number
| The name of a cargo that is particularly dangerous for transportation | room
UN |
Class
ADR |
| ZINC - POWDER or ZINC - DUST | 1436 | 4.3 |
| Zinc Nitric Acid Zinc Nitrate | 1514 | 5.1. |
| Zinc dichromate zinc dichromate | 3087 | 5.1. |
| Zinc manganese zinc PERMANGANATE | 1515 | 5.1. |
| Zinc chloride chlorine ZINC CHLORIDE ANHYDROUS | 2331 | 8 |
| Zinc-alkyldithiophosphate | 3082 | 9 |
| Zincaryldithiophosphate | 3082 | 9 |
| ZINC ARSENATE, ZINC ARSENITE or ZINC ARSENATE AND ZINC ARSENITE MIXTURE | 1712 | 6.1. |
| ZINKA BROMATE | 2469 | 5.1. |
| Zinc bromide | 3077 | 9 |
| Zinc hexafluorosilicate Zinca Fluorosilicate | 2855 | 6.1. |
| Zinc hydrosulphite Zinca Dithionite (zinc hydroxide) | 1931 | 9 |
| ZINC DITHIONITE (ZINC HYDROSULPHITE) | 1931 | 9 |
| Zinc dichromate | 3087 | 5.1. |
| Zinc Nitrate | 1514 | 5.1. |
| ZINC PERMANGANATE | 1515 | 5.1. |
| ZINC PEROXIDE (peroxide) | 1516 | 5.1. |
| ZINKA RUBBER | 2714 | 4.1 |
| ZINCAS PHOSPHIDE | 1714 | 4.3 |
| ZINC PEROXYLICATE | 2855 | 6.1. |
| Zinc Chlorate | 1513 | 5.1. |
| ZINC CHLORIDE ANHYDROUS | 2331 | 8 |
| ZINC CHLORIDE SOLUTION | 1840 | 8 |
| ZINC CYANID | 1713 | 6.1. |
| ZINC-AMMONIUM NITRITE | 1512 | 5.1. |
| GALLIUM | 2803 | 8 |
- Ghetchellit - "New Almaden blend" - arsenide and antimony sulfide (modern sulfosol)
- Antimony is a toxic metal (semimetal) , widely used in metallurgy, medicine and engineering
- Zirconium - a rare and undiscovered metal and the most dangerous precious stone in oxide and salt
- Gold - yellow dangerous and poisonous metal of modern accurate digital and cable technologies
- Sulfur is a golden-yellow toxic substance and a sign of active volcanic activity
- Cadmium is an undisputed toxic silvery metal unknown to a wide range of people
- Lead - a toxic gray imitator of metallic silver and toxic metal blende
- Arsenic is a classic poison of medieval and modern poisoners and medicine in medicine
Poisonous and radioactive dangerous stones and minerals
** - poisonous stones and minerals (mandatory check in the chemical laboratory + explicit indication of toxicity)
** - radioactive stones and minerals (mandatory check on the standard dosimeter + ban on open sales in case of radioactivity exceeding 24 milli / g / h + additional measures of population protection)
Catalog of minerals and semi-precious stones of the world by groups
** - poisonous stones and minerals
** - radioactive stones and minerals


Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.