|
section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
FIT as well as technology chromium metal
![]()
See also: |
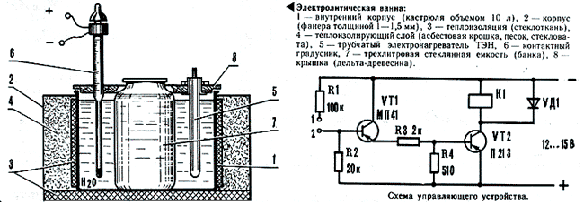
Chrome, one of the necessary practical dvigatelisty coatings, is among the most time-consuming process of electroplating. It requires special care and cleanliness as when preparing the electrolyte, as well as the substances themselves, included in its composition. Distilled water is used, or (as a last resort!) Thoroughly boiled. Making bath Classes start with a model electroplating baths manufacturing, just pick up a pre-pan 10 liters and a three-liter glass jar. Tanks smaller is better not to use - it can complicate the adjustment of process parameters, of course also when the above values bath tank is only enough only for 6-8 chrome plating cylinder liners. Gluing of 1-1.5 mm plywood housing, according to collect the bath shown in the drawing and close all plywood ring. Work on the bathroom ends vytachivaniem cover the pan and installation of heaters on it as a contact thermometer. Today - electrical equipment. For feeding bath mozhnoispolzovat just a constant current source connected to the output electrolytic capacitor 80,000 microfarads X 25 In. Feeding wires must own cross-section not less than 2.5 mm 2. Turn the amperage, voltage regulator replacement may serve the sectional rheostat. He turned on a row with a galvanic bath also consists of parallel, single-pole knife switches included sections. Each subsequent opposition has twice the previous one. The number of sections 7-8. The front panel of the unit set litany two outlets 15 A, single - normal polarity, the other backward. This will quickly spend the anode parts finish and go to chrome fork unsophisticated rearranged. Sockets with three outputs to not make a mistake in polarity (connected, of course, only a pair of jacks). To maintain a constant temperature of the electrolyte bath is equipped with a contact thermometer. Direct control of heaters work he can not because of the impressive currents therefore need to build a simple device, whose scheme is shown in the figures.
Details of thermostat: transistors VT1 - (MT 13 - MP16, MP39, MP42); VT2213-217 (P213-P217) with any letter designations; Resistors - (MLT-0.25); LED - (D226, D202, D205); relay - (TKE 52 PREP or RF4.530.810 IPOs passport). Adjusting the thermostat: if the shorted points 1-2 turnip does not work, connect the emitter and collector VT1. Enabling relay indicates a fault or a small gain VT1. Otherwise faulty transistor VT2 or he has insufficient gain. Gathering also established way of baths, mozhnopristupat electrolyte preparation. To do this: The last operation is necessary for the accumulation of a small number of Hmong Cr 3 (2-4 g / l), which stay friendly impact on the process of deposition of chromium. The composition of the electrolyte Chromic anhydride 250 g / l or 150 g / l
modes chroming chroming process strongly depends on the temperature of the electrolyte and the current density. Both factors act on the outer appearance and properties of the coating, but it appears to chrome output current. You must not forget that with increasing temperature the current output is reduced; with increasing current density increases current efficiency; at lower temperatures and a constant current density obtained gray coat, but at high - dairy. A practical route found the best chroming treatment: current density of 50-60 A / dm 2 at an electrolyte temperature of 52 ° - 55 ° ± 1 °. To ensure efficiency of the electrolyte in the bath prepared mozhnopokryt a few details such as the shape and size of the working samples. Picking up mode is also learning the output current metering unsophisticated sizes up to and after chroming, mozhnopristupat to cover sleeves. According to the proposed method is applied to the chromium steel, bronze and brass parts. Their preparation is washing the surfaces to be chromium plating, and then soap gasoline (with a toothbrush) in hot water charge as a mandrel placed in the bath. After dipping in the electrolyte have to wait 3-5 seconds and then turn on the operating current. The delay is necessary to ensure that the item is warmed. Immediately comes the activation of the surface of the parts made of brass and copper, as if these metals are well etched in the electrolyte. But more than 5 seconds delay can not be - there is zinc, which stay in the electrolyte is not allowed in the composition of these metals.
Chrome-plated aluminum alloy In the process of applying chromium on aluminum alloys have to stay apart. Implementation of such coatings prkticheski is always fraught with difficulties. Previously it only need pre-coating of the intermediate layer. Aluminum alloys containing an impressive number of silicon (up to 30%, alloy grades AK12, AL25, AL26, CAC-1), mozhnohromirovat follows:
Other activity, if you want to cover AK4-1 chromium alloy. It manages othromirovat only through the intermediate layer. These methods include: zincate treatment; nickel sublayer; through the nickel salt; trim items through the anode in phosphoric acid solution. In all cases, the items are prepared as follows:
Etching using sodium hydroxide solution (50 g / l), time to finish at a temperature of 10-30 with a solution of 70-80 °. For the etching of aluminum alloys containing manganese and silicon, it is better to use such a solution, in parts by weight:
The intermediate coating galvanizing
If the zinc coating is laid down unevenly, the item is lowered into the bleed 50-percent solution of nitric acid at 1-5 with and later repeated washing Galvanizing. For magnesium-aluminum alloy dual Zinc is necessary. Causing another layer of zinc, washed item, a charge mandrel also energized (no zinc supply voltage has time to dissolve in the electrolyte partly, contaminating it) installed in the bath. Pre mandrel with the workpiece immersed in a glass of water heated up to a temperature of 60 °. The process of chroming normal. Nickel plating (Chemical)
Last operation - in a solution with the following composition: nickel sulfate 30 g / l, sodium hypophosphite 10-12 g / l sodium acetate 10-12 g / l, glikokol- 30 g / l. He first component without hypophosphite-ta, which is introduced before the nickel plating (with hypophosphite solution continuously not stored). Temperature nickel plating solution at 96-98 °. It can be used as a solution without glycine, while the temperature should be lowered to 90 °. For 30 minutes on the workpiece is deposited nickel layer thickness of 0.1 up to 0.05 mm. Dishes for work - only a glass or porcelain, as if nickel is deposited on all metals of the eighth group of the periodic table. Good yield nickel plating brass, bronze and other copper alloys. After the deposition of nickel a heat treatment to improve the adhesion to the base metal (200-250 °, the shutter speed 1-1.5 hours). The part is mounted on the mandrel for chroming also lowered to 15 to 40 with a solution of 15% sulfuric acid, where the return current is processed at the rate of 0.5-1.5 A / dm 2. There is activation of nickel, oxide film is removed, and the coating becomes gray. The acid should be used only chemically pure (in the worst case the battery). Another way Nickel acquires black paint and chrome on the surface of any such life does not lie. Thereafter, the mandrel with the workpiece is loaded into a bath of chromium plating. Initially allow current to couple more times, and then for 10-12 minutes until it reduces worker. Defects electroless nickel:
Removal of nickel aluminum alloys mozhnoproizvodit in nitric acid solution. Another time, in the process of self-discharge nickel stems - loss of powdered nickel. In this case, the solution is poured, but the dishes treated with nitric acid solution to remove nickel from its surface, which will hinder the deposition on the workpiece. It should be noted that the nickel-phosphorus personally by itself has very interesting properties, not inherent in chrome plating. This uniform layer on the surface of parts (not required after finishing the deposition); high hardness after heat treatment (400 ° mode for an hour alienates HV 850-950 hardness of the coating and more); low coefficient of friction compared with chromium; very slight expansion; high tensile strength. Nickel-phosphorus without further application of chromium can be used not only as an intermediate coating on the sleeves, but also as business, reducing friction and wear, for spools and piston pins. After a couple years of active operation of the engine with the details of such a finish on them lacked a clear development, characteristic of red-hot steel surfaces. Applying through chromium nickel salt
Chrome Application through anodic treatment
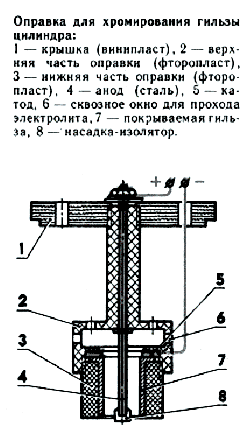
After finishing the anode should be performed momentary cathodic furnish in an alkaline bath which removes the oxide layer partly. Studies have shown that in the process of finishing aluminum alloys anode in phosphoric acid is formed on the rough surface parts, which promotes lasting cohesion subsequently applied coating. Adaptations, mandrel Chrome-plated sleeve
Anode - steel stud; from one end thereof over a length of 50-60 mm is welded with lead antimony (7-8%). Lead eat through the outside diameter of 6 mm (for working sleeves of 0.15 mm). On the other hand threaded studs for fixing the wire. The cathode is a ring with an internal diameter of 0.5 mm greater than the internal size of the liner. It vchekanivaetsya section of insulated wire. Copper and brass wires is better not to use - electrolyte dissolves them, ikontakt may be broken. Before installing the plug in the bath is useful to check the strength of the tester contacts. Chrome-plated steel parts
Rated current is determined by multiplying the area of the surface of the process hromiruemoy current. To become the last dimension of 50 A / dm 2. When plating, for example, planting space under the main bearing to the crankshaft of the engine KMD-2,5 rated current will be equal to 0.03 dm 2 x 50 A / dm 2 x 1,5 A. For chrome plating crank pin will need a new mandrel. As well as the decoration of the crankshaft, all exposed areas of the surface of the closed glue "AGO". The anode is machined from steel followed by casting the lead as boring holes for thumbs. The use of a steel part due to the need to provide safe contact - in lead screw connections unreliable. current calculations are similar. Work carried out in the mandrel shaft via a special nozzle. Almost nothing is no different chrome bearings. The only thing - to protect the internal elements of the parts it is filled with grease or other grease which later coating is washed with petrol.
Tool for chroming outer race ball bearing:
Chrome plating defects and their reason
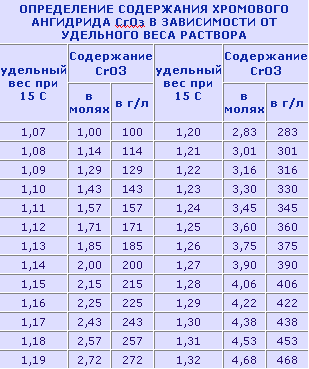
The concentration of chromic anhydride in the electrolyte is controlled by a hydrometer. The concentration of sulfuric acid is only possible to determine, unfortunately, indirectly, on the quality of the coating. In the process of chroming moves electrolyte evaporation. In these cases, water is added up to the desired level. This is done without installing parts - you can change the temperature of the electrolyte. After chrome plating, all products subjected to heat treatment for 2-3 hours to remove hydrogen, at a temperature of 150-170 °. All labor carried out under an extractor device, rubber gloves and glasses. According to the magazine "Model Builder". Source №5 for in 1989.  Liked? Subscribe to RSS news!
You can also support shram.kiev.ua, click: Do not be amiss to your friends and find out this information, share with them the article! |



Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.