| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2158048
![]()
SOLAR ENERGY TRANSFORMER TO ELECTRICAL
The name of the inventor: Grishin VK (RU); Evening Alim Alexandrovich (BY); Sinyavsky VV (RU)
The name of the patent owner: Open Joint-Stock Company "SP Korolev Rocket and Space Corporation Energia" (RU)
Address for correspondence: 141070, Moscow Region, Korolev, ul. Lenina 4a, RSC Energia after SP Korolev, Industrial Property and Innovation Department
The effective date of the patent: 1999.02.05
Purpose: autonomous sources of electricity, including space.
SUMMARY OF THE INVENTION: The solar energy converter comprises a sealed enclosure divided into two compartments, filled with the same dissociating diatomic gas and separated by an ion-exchange membrane, the material of which is an electrolyte with ionic conductivity over the dissociated atomic gas of the diatomic gas, on both side surfaces Membranes are gas-permeable electrodes provided with current leads, one of the compartments being provided with a heat removal system. The part of the shell of the other compartment is made of a material transparent for solar radiation, and as a diatomic gas a gas dissociating into an atomic gas under the influence of solar radiation is chosen. The invention allows direct conversion of solar energy into electrical energy.
DESCRIPTION OF THE INVENTION
The invention relates to electric power sources with direct conversion of heat to electricity and can be used in the creation of autonomous solar power sources, including space applications.
There are known machine and direct converters of solar thermal energy into electrical [1]. Machine converters include steam turbine and gas turbine units, as well as internal combustion engines, Stirling engines, piston expansion machines. The main types of direct heat converters are thermoelectric, thermionic and magnetohydrodynamic. In addition to the heat converters considered, other primary energy converters are also known, such as chemical fuel cells or electrochemical generators and light photovoltaic cells.
A converter in the form of a hydrogen-oxygen fuel cell is close to the invention [2]. The converter consists of two compartments, separated by an ion-exchange membrane, to the side surfaces of which are pressed electrodes made in the form of a grid. The electrodes are connected to the current collectors. On one side of the membrane is hydrogen, on the other - oxygen. On the side of the oxygen electrode, there are wicks to drain the water that is formed and the tubes in which the cooling water circulates. The hydrogen and oxygen compartments are not connected to each other. All this is inside the case. Separate such elements are electrically commuted to a battery of fuel cells.
Such an element is a converter with a flow rate of the working fluid, which leads to a limited resource and energy intensity.
The closest to the invention in terms of the totality of technical features is a thermal energy converter directly into an electrical one [3] containing a sealed enclosure divided into two compartments filled with the same dissociating diatomic gas and separated by an ion-exchange membrane, the material of which is an electrolyte with ionic conductivity On the dissociated atomic gas of a diatomic gas, gas permeable electrodes are provided on both lateral surfaces of the membrane, equipped with current leads, one of the compartments being provided with a supply system and the other with a heat removal system.
Such a device is a converter of thermal energy directly into electrical energy, but it can not be used for directly (without preliminary conversion into heat) conversion of solar energy directly into electrical energy.
The technical result achieved by using the invention is to enable direct conversion of solar energy into electrical energy.
This technological result is achieved in a solar energy converter into an electrical one, comprising a sealed housing divided into two compartments, filled with the same dissociating diatomic gas and separated by an ion-exchange membrane, the material of which is an electrolyte with ionic conductivity over the dissociated atomic gas of a diatomic gas at Both gas side surfaces of the membrane are gas-permeable electrodes provided with current leads, one of the compartments being provided with a heat removal system in which a part of the shell of the other compartment is made of a material transparent to solar radiation, and a gas dissociating into an atomic gas under the action of a solar Radiation. A gas with a low dissociation energy, for example iodine, fluorine, chlorine, bromine or mixtures, can be chosen as the diatomic gas dissociating under the action of solar radiation. The heat removal system can be made in the form of a heat radiating system, for example, based on heat pipes.
The diagram shows the scheme for converting solar energy into electrical energy.
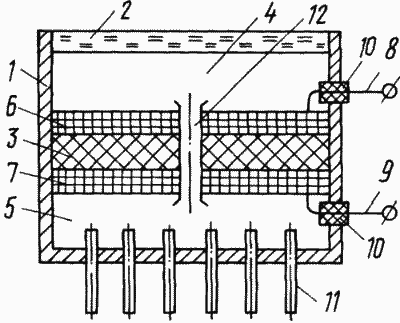 |
The solar energy converter comprises a housing 1 with two compartments, the housing part 2 of one of the compartments being made in the form of a transparent wall for solar radiation, for example glass, quartz or other transparent material. The ion exchange membrane 3 divides the inner space within the body 1 into two compartments - irradiated with solar radiation 4 and cooled 5. On both sides of the membrane 3 are placed contacting gas permeable electrodes 6 and 7, for example in the form of a grid, each of which is provided with insulated from the housing 1 8 and 9, which are separated from the enclosure 10 through the sealed lead ducts 10. The compartment 5 is provided with a heat removal system 11 which can be made heat-radiating, for example on the basis of heat pipes or in the form of heat-radiating ribs. The compartments 4 and 5 are filled with a diatomic gas, for example, iodine. The compartments 4 and 5 communicate with each other, for example, in the form of a tube, a slot, a capillary 12 in an ion-exchange membrane, or as a separate unit, which can be configured as a throttle or a check valve. |
SOLAR ENERGY TRANSFORMER TO ELECTRICAL
WORKS AS FOLLOWING
Solar radiation, passing through the transparent part 2 of the body 1, gets on the molecules of the diatomic gas sorbed by the surface of the electrode 6 in the compartment 4. Upon irradiation, the diatomic gas dissociates into an atomic gas with absorption of a certain amount of solar energy proportional to the specific heat of dissociation of the selected diatomic gas. The atomic gas formed as a result of dissociation in compartment 4 has a higher chemical potential than the gas in compartment 5 in the molecular state (at equal pressures). Due to this difference in chemical potentials, it is possible to obtain electrical work if they are separated by an ion-exchange membrane 3, the material of which is a solid electrolyte containing ions that can be obtained by ionizing a gas atom (eg iodine) by attaching an electron to it. Then, if from the side of the atomic gas the surface of the membrane (electrolyte) is in contact with the electronic conductor (electrode 6), the atoms of the gas will capture the electrons of the electronic conductor and pass in the form of ions into the electrolyte (membrane 3). If the other side of the membrane (electrolyte) is in contact with the electron conductor (electrode 7), but this side is the molecular gas, then due to its lower chemical potential than that of the atomic gas, the ionization of the molecular gas will occur to a lesser extent. As a result, the electron conductor (electrode 6) on the side of the atomic gas has a more positive potential than the conductor (electrode 7) on the molecular gas side. Therefore, when the conductors (electrodes 6 and 7) are closed through an external circuit, an electric current will flow. In addition, the concentration of ions is higher on the side of the membrane (electrolyte) 3, contacting with the atomic gas (illuminated compartment 4), therefore, when the electrodes 6 and 7 close inside the membrane (electrolyte) 3, a diffusion ion current arises. As a result of this process, the working substance is transferred from the part of the system where its chemical potential is higher (illuminated compartment 4), to the part where its chemical potential is lower (compartment 5 with molecular gas). Therefore, when the electrodes are closed through an external circuit, the gas pressure in the cooled compartment 5 will increase, and in the illuminated compartment 4 decrease. To organize a continuous process of generating electricity, it is necessary to ensure the transition of the diatomic gas from the cooled side of the membrane (compartment 5) to the illuminated (compartment 4). This is realized by connecting the compartments 4 and 5 by means of a tube 12, which can be made in the form of a check valve or a throttle. The unconverted part of the solar radiation supply is discharged by the heat removal system 11, which can be made on the basis of heat pipes or in the form of heat transfer ribs.
Thus, part of the solar energy expended on the dissociation of a diatomic gas into an atomic gas, then by recombination of an atomic gas with the help of an ion-exchange membrane, becomes electricity.
CONSIDER THE PROCESS OF TRANSFORMATION MORE
Let the diatomic gas X2 under the influence of solar (electromagnetic radiation) with a frequency wg and higher dissociate into an atomic
![]()
The dissociating gas absorbs the following amount of solar energy per square meter of the irradiated surface (with full absorption)
![]()
Where A is the solar constant; T is the temperature of an absolute black body with a spectral energy distribution close to that of the Sun (6000K); H is the Planck constant.
Equation (2) is obtained from Planck's formula for the spectral density of radiation of an absolutely black body.
As a diatomic gas, it is advisable to choose halogen, since other diatomic gases have too high values of wg. According to the known data on wg, the coefficient K and the fraction of energy absorbed during the dissociation of halogens were calculated (Table 1).
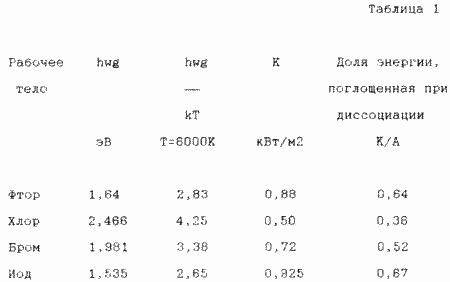
Among the halogens, iodine is the best.
The resulting atomic photodissociation has a higher chemical potential than in the molecular state at equal pressure. Due to this difference in chemical potentials, it is possible to obtain electrical work in the following way.
Let the atomic gas come into contact with an electrolyte (an ion-exchange membrane) containing ions that can be obtained by ionizing an atom X by attaching an electron e to it. Then, if from the side of the atomic gas the surface of the electrolyte (membrane) is in contact with the electronic conductor (electrode) in addition, the atoms X will capture electrons of the electronic conductor and pass in the form of X-1 ions into the electrolyte according to the scheme

If the other side of the electrolyte (membrane) is in contact with the electron conductor (electrode), but on this side the molecular gas X2 is located, then due to its lower chemical potential than the chemical potential of the atomic gas, the ionization process X2 according to scheme
![]()
Occurs to a lesser extent. Thus, the electronic conductor from the side of the atomic gas has a more positive potential, i.e. When the conductors are closed through an external circuit, electric current will flow. In fact, the proposed device is a galvanic cell (GE), which can be schematically illustrated as follows:
![]()
Let us estimate the expected characteristics of the proposed converter. We choose the pressure of the working fluid equal to 1 bar (105 Pa). Then the EMF E of the converter as GE will be equal to
E = D Go / 2F,
Where D Go is the standard isobaric potential of the dissociation reaction X2 ---- 2X, F is the Faraday number (96500 ppm / g-eq). According to the reference data, we have the data presented in Table. 2.
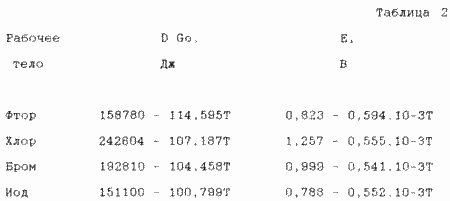
Calculated using the data of Table. 2 values of the EMF E and the electrochemical efficiency h, which is obtained by dividing E = A + BT by the constant term A in the equation E = A + BT, are given in Table 3.
The total efficiency of the converter can be characterized by the maximum possible value of the electric power from the unit of the irradiated surface and the overall efficiency of the energy conversion, which is equal to the product of the electrochemical efficiency h by the fraction of energy absorbed during dissociation (Table 1)
Wmax = Kh (kW / m 2 ),
H total = Kh / A
The values of wmax and h are generally given in Table. 4.

Thus, the converter of solar energy to electrical has a relatively high efficiency and in principle can have a long life in connection with the lack of consumable components. To increase efficiency, a gas-permeable electrode (electronic conductor) should be made with increased sorption capacity with respect to a diatomic gas.
INFORMATION SOURCES
A.A. Kulandin, S.V. Timashev, V.P. Ivanov. Energy systems of space vehicles, - M .: Mechanical Engineering, 1972, p. 10 - 15.
Power plants of space vehicles. Podshivalov, E.I. Ivanov, L.I. Muratov, etc. Under the general. Ed. Nevyarovsky and V.S. Viktorova. -M .: Energoizdat, 1981, p. 18-19, 27-29.
Patent RU 2074460 C1 "Thermal energy converter directly into electrical" / В. K. Grishin, A.A. Evening, V.V. Sinyavsky / Application 94039447 of 04.10.94.
CLAIM
The converter of solar energy into electrical, containing a sealed enclosure divided into two communicating compartments filled with the same dissociating diatomic gas and separated by an ion-exchange membrane, the material of which is an electrolyte with ionic conductivity over the dissociated atomic gas of a diatomic gas, on both lateral The gas-permeable electrodes provided with the current leads are located on the membrane surfaces, one of the compartments being provided with a heat removal system, characterized in that the part of the housing of the other compartment is made of a material transparent for solar radiation, and a gas dissociating into an atomic gas under the influence of solar radiation is selected as a diatomic gas .
The invention according to claim 1, characterized in that a gas with a low dissociation energy is selected as the diatomic gas dissociating under the action of solar radiation.
The invention according to claim 2, characterized in that iodine, fluorine, chlorine, bromine or mixtures thereof is selected as a diatomic gas with a low dissociation energy.
print version
Date of publication 05.11.2006гг




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.