| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2200735
![]()
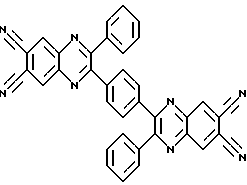
2,2'-diphenyl-3,3 '- (N-phenylene) bis (6,7-HINOKSALINDIKARBONITRIL
Name of the inventor: Ivanovo SA .; Smirnov AV .; Abramov IG .; Abramova MB .; Shamshin SV .; Plahtinsky VV
The name of the patentee: Yaroslavl State Technical University
Address for correspondence: 150023, Yaroslavl, Moscow Avenue, 88, YAGTU, Intellectual Property Department.
Starting date of the patent: 2001.10.03
The invention relates to the preparation of new heterocyclic tetranitrilov tetracarboxylic acids of the formula I, which can be used to obtain poligeksazotsiklanov - fluorophores bifluoroforov, trifluoroforov. Such poligeksazotsiklany promising for use as active media of solid and liquid lasers, scintillators, especially for indication of hard radiation, for the transformation of short-wave radiation into long-wave transmission of information via fiber-optic lines, to increase the power of solar panels to protect the securities for manufacture of billboards.
DESCRIPTION OF THE INVENTION
The invention relates to the production of new tetranitrilov heterocyclic tetracarboxylic acids which may be used to obtain not previously described poligeksazotsiklanov - fluorophores bifluoroforov, trifluoroforov. Such poligeksazotsiklany promising for use as active media of solid and liquid lasers, scintillators, especially for indication of hard radiation, for the transformation of short-wave radiation into long-wave transmission of information via fiber-optic lines, to increase the power of solar panels to protect the securities for production of billboards, etc.
A known compound - piromellitnitril, which, together with unsubstituted rhodamine used for fluorescent poligeksazotsiklanov (Siling SA, Theophany BN, NN Barashkov etc. Poligeksazotsiklany based heterocyclic diamine // Polymer B. Comm.... - 1988. - V. 30. - 4 - pp 286-291).
The resulting poligeksazotsiklan has the following indicators: the emission band at 532 nm with excitation at 312 nm band.
and known heterocyclic tetrakarbonitrily (Ivanovo SA Abramov IG, Smirnov AV, etc. 2,3,7,8-tiantrentetrakarbonitril // Pat. 2,165,417 RF, BI 11, 2001 .; Ivanovsky SA Abramov IG, Smirnov AV et al. 2,3,7,8-fenoksatiintetrakarbonitril // Pat. 2,165,418 RF, BI 11, 2001). Poligeksazotsiklanov spectral characteristics obtained based on data and unsubstituted tetrakarbonitrilov Rhodamine are shown in the table (see. The end of the description).
Poligeksazotsiklan obtained on the basis of 2,2-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitrila), claimed in this patent has unique spectral characteristics different from the characteristics already known geksazotsiklanov.
Varying in tetrakarbonitrile, various substituents and bridging pieces can be paired with a specific diamine more accurately adjust the maximum end poligeksazotsiklana radiation, ie, as close as possible to the implementation of the specified properties. In this way, laser dyes perenastraevyemyh lasers to the required length and variable absorption wavelength of radiation waves can be received, and all becomes available colors of fluorescent dyes used in a wide variety of purposes.
Object of the present invention is to provide a 2,2'-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitrila).
The inventive 2,2-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitril) of formula
This compound was prepared by a condensation reaction of excess 4,5-double diaminoftalonitrila with 1- [4- (2-oxo-2-phenylacetyl) phenyl] -2-phenyl-1,2-etandionom scheme.
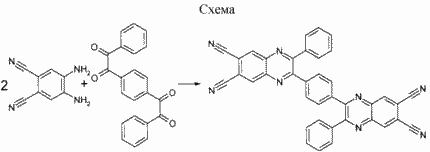
The reaction is conducted in acetic acid at reflux temperature for 1-1.5 hours.
The invention is illustrated by the following examples.
Example 1. In a flask equipped with a reflux condenser is charged successively with 30 ml of acetic acid, 3.2 g (0.02 mol) of 4,5-diaminoftalonitrila, 3.4 g (0.01 mol) of 1- [4- (2-oxo- 2-phenylacetyl) phenyl] -2-phenyl-1,2-etandiona. The reaction mixture was kept at reflux for 1.5 hours. The reaction mixture was poured into 100 ml of water, the precipitate formed is filtered off, washed with 50 ml of water and crystallized from DMF.
Obtained 4.82 g (82.2% of theory) of 2,2-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitrila) - white crystalline powder with mp.. > 300 o C.
Found,%: C 77.77; H, 3.12; N 19,03
Calculated,%: C 77.80; H, 3.10; N 19,10 (C 38 H 18 N 8)
1H NMR ([2N6] DMSO): ![]() ppm .: 8,90 (s, 4H), 7,45 (m, 14H).
ppm .: 8,90 (s, 4H), 7,45 (m, 14H).
Example 2. Condensation of 2,2-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitrila) with rhodamine 123. In a flask equipped with a stirrer, thermometer, reflux condenser and argon capillary entry charged 5.7 g (0.01 mole) of 2,2-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitrila), 3.8 g (0.01 mole) of rhodamine 123 and 10 g of phenol. The resulting mixture was slowly heated with stirring to 175-185 o C. The resulting melt is kept under stirring under argon until no ammonia. After completion of the reaction, the reaction mixture was poured into 20 ml of ethanol, the precipitate was filtered off, washed with 3 ml of ethanol and dried at T = 60 o C for 2 hours, then at vakuume over P 2 O 5. To give 12.0 g (96% of theory) poligeksazotsiklana.
Found,%: C 76.98; H, 4.09; N 11,18
Calculated,%: C 77.02; H, 4.05; N 11,23 (C 80 H 50 N 10 O 6)n
In poligeksazotsiklana IR spectrum no band 2220 cm -1 -C ![]() N, strip 680 is present cm -1 -C = N.
N, strip 680 is present cm -1 -C = N.
Structural formula poligeksazotsiklana obtained based on 2,2-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitrila) and rhodamine 123
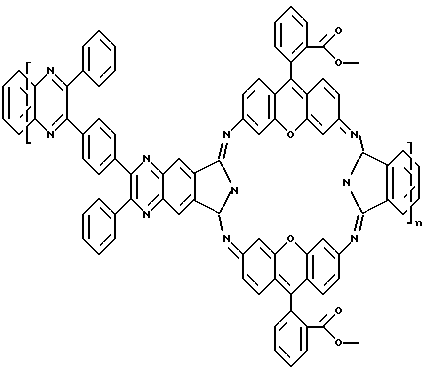
Poligeksazotsiklan obtained on the basis of 2,2-diphenyl-3,3 '- (p-phenylene) bis (6,7-hinoksalindikarbonitrila) and rhodamine 123 has the following spectral characteristics: a maximum of the emission spectrum - 448 nm at the maximum absorption spectrum - 280 and 365 nm.

CLAIM
2,2-biphenyl-3,3'- (p-phenylene) bis (6,7-hinoksalindikarbonitril) of formula
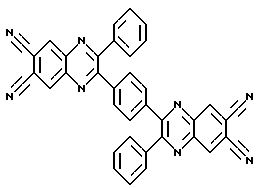
print version
Publication date 03.02.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.