|
section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2162991
![]()
Geothermal systems for generating electricity
Name of the inventor: James Schnell (US)
The name of the patentee: Schnell James (US)
Address for correspondence: 103062, Moscow, ul. Pokrovka, 27, Building 1 AG, cooperative intellectual property agency "INTELS" Dyakonova OM
Starting date of the patent: 1996.06.07
The system is designed to produce electricity by converting geothermal energy. Conversion is performed by the endothermic reactions in the bottom of the well for capture and storage of geothermal heat and exothermic reactions at the wellhead for the release of heat stored in the products of endothermic reactions. In one preferred embodiment of the present invention the endothermic reaction is a reaction of water decomposition. To activate the endothermic reaction, and for the production and separation of the resulting products using a catalytic device in which each product type is selectively diffused into a separate corresponding channel. Endothermic products undergo exothermic combustion in the turbine, and the products of the exothermic reaction in the condensed immediately refrigerated. In one preferred embodiment of the invention a refrigerator steam condenses into liquid water to return to the wellbore, providing thereby obtaining a closed cycle. The invention allows for the efficient conversion of geothermal energy into electricity.
DESCRIPTION OF THE INVENTION
The present invention relates to a power generation by utilizing geothermal heat, and more particularly to the use of geothermal heat to produce products of endothermic reaction by a catalytic device or electrolytic reaction by a thermocouple.
The standard systems for the conversion of geothermal energy into electricity using hot water or other liquid to vapor in the earth's crust; then steam for power generation is supplied to the turbine. Typically, the geothermal heat is applied to the surface via wells that are recessed in the porous rocks containing steam or brine that circulate in the earth's crust at a depth sufficient to capture a significant amount of heat. An example of such a system is described in US patent N 3786858 (1974).
However, modern steam turbines operate most efficiently at very high temperatures, substantially higher than the temperature achieved in the porous rock containing steam or brine that is usually used for power generation by a geothermal power plant. Achievable for practical purposes, the deep heat of the Earth has an insufficient concentration. For this reason the steam turbine operated by means of geothermal energy, are less efficient. During operation they are limited to what depth the heat extracted from the crust can not be used thereafter. This heat must be used immediately or lost.
Also, brine or steam loses a significant amount of its heat (generally 25% or 30%) to the extent of delivery to the surface. Brine or steam from geothermal porous rocks are usually accompanied by hydrogen sulfide and other undesirable gases, which must be captured before they escape into the atmosphere. Since the temperature of the brine or steam is relatively small, of sufficient magnitude to generate electricity to be transported to the surface of a large number of them. Consequently, there is a need for large-diameter boreholes, drilling cost is high. Moreover, saline, or water vapor, which is fed to the surface is often highly mineralized and corrosive. If it is used directly in a turbine, then to withstand such conditions, such a turbine to be modified, and in accordance with this further reduces system efficiency. Alternatively, the brine or steam through the heat exchanger in a binary generating system can be used to boil another fluid. Due to the heat exchanger, and in this embodiment, there are additional efficiency losses.
Another problem that may be caused by the minerals in the brine and water vapor, is scaling in the wells, which could accumulate over time and must be periodically removed. Brine has disposal problems after it has been used, unless it is subjected to re-injection into the porous rock, which require expensive injection pump and may cause contamination of the porous rock. Even if the brine is subjected to a second injection, some of the salts may precipitate out of solution when the salt solution is cooled prior to reinjection. These salts, which may be radioactive or otherwise hazardous, must be safely removed and disposed of.
The biggest limitation is that there is very little porous rocks that have sufficient and sufficiently heated to provide a perspective of the use of geothermal energy from an economic point of view.
It is currently conducting a feasibility study drilling in the hot dry rock and pumping water to create a geothermal porous rock, which can then be attached to generate electricity. However, such systems have the same problems as conventional geothermal systems and are more expensive. Systems of the prior art which utilize a hot dry rock, requires drilling two wells (injection well for injecting water to create a reservoir and a separate production well for continuously supplying steam to the surface). The use of only one well for injecting water and removing the water vapor will not be effective, since either too much energy would be lost when the injected water passes through the rising steam, or the steam would be retrieved only intermittently (discontinuously), so that the thermal energy will not be fed to the generator constantly.
Injection of water into the rock requires energy, which is a significant fraction of the energy that the system can produce, thus reducing the efficiency of the system. A quantity of water which is pumped, and is lost in the cracks in the rock and returns to the production well. The greater the pressure which is applied to drive the water from the injection well to the production well, the more water is lost. The higher pressure in the injection well causes the cracks to expand (increase), as does the colder water, which causes the compression of the rock. Expansion must in the production well, where it accelerates the release of energy in the rock. Tests have shown that short-term closing of a production well improves its overall efficiency due to an increase in its expansion.
At an early stage of development of the geothermal power generation technology predominant method used to generate electricity, and the burning of hydrocarbons would transform the resultant heat to electricity. Until the last decade, most of the electricity is generated by burning coal to produce steam. Recently, approximately half of all new generated electric power obtained by combustion turbines that burn oil or natural gas, using their capacity to generate electricity through a direct connection with the generator. In a system in which an "combined cycle" heat of the combustion turbine exhaust is used to generate steam, which is then converted by the steam turbine in the additional electricity. However, the internal combustion turbine uses a significant amount of the energy it generates, for compressing air, which is introduced in order to maintain its operation. Each of the above combustion process leaves significant amounts of nitrogen oxides, which create pollution and a prerequisite for acid rain. They form carbon dioxide, thus contributing to global warming. If used as a fuel oil or coal, it is released into the atmosphere, and sulfur dioxide (sulfur dioxide), which may further lead to acid rain, and dust particles and excreted. During the combustion of coal ash is formed, and which must be properly disposed of. In addition, all these processes deplete limited natural resources.
Other technologies used to generate electricity include the production of electricity in nuclear power plants, hydroelectric power plants, but also by using solar and wind energy. for hydroelectric power generation, but also when using solar or wind energy is limited in time and space according to the areas in which they are effective in causing Accordingly, the need for effective trapping systems and influencing the environment. In addition, power generation using solar and wind energy much more expensive than conventional technology.
Many energy generated in the present time are obtained by condensing steam turbines. Fuel is burned in the combustion chamber and release exhaust gases into the atmosphere, while the heat produces superheated steam. The steam passes through a steam turbine generator for generating electricity and is condensed at the end of the cycle. The pressure drop due to condensation at the outlet end of the turbine enables the turbine to rotate more freely, but the efficiency of the overall process is still less than 40%, partly because of the need to convert the combustion heat into steam energy. A significant amount of energy and is lost along with the combustion exhaust gases.
The ever-increasing share of new generating capacity, introduced in recent years, carried out thanks to the internal combustion turbines. Internal combustion turbines use the energy released from fuel combustion, for rotation of the turbine shaft, which then drives the electric generator. Such a combustion turbine requires a large volume of air to be filtered, and often heated or cooled. This leads to the introduction of pollutants into the turbine and to the energy consumption. The exhaust gas which is released into the atmosphere carries a significant amount of energy, and contamination. Furthermore, the internal combustion turbine uses a significant amount of energy to compress the air flow, but only 16% (or less) of oxygen used in the combustion process.
Only recently combustion turbines achieved efficiencies approximating a numerical value by 40%, when operating in the "simple cycle". Efficiencies approaching on the numerical value of 50% can be achieved by combustion turbines operating in "combined cycle", in which exhaust heat from the combustion turbine is converted into the energy of the steam which is then used to drive a generator a steam turbine. However, the steam is not superheated steam, which is typically used to actuate the steam turbine generators. Consequently, the steam cycle system of a combined cycle is less efficient than a simple steam turbine.
As a steam turbine and internal combustion turbines (simple or combined cycle) lead to contamination of the environment obtainable in the allocation to the atmosphere products or by-products of combustion. They lose their effectiveness due to release the exhaust gases from the considerable amount of energy of the combustion chamber together. The steam generator and the combustion turbine generator lose efficiency combined cycle heat conversion due to water vapor pressure.
The present invention provides a system for efficient conversion of geothermal energy into electricity, in which one or more substances are transported through the well to a depth at which depth the heat of the Earth (whether it is the heat of porous rocks containing brine or steam or hot, dry rock) is sufficient to cause a thermal reaction, such as an endothermic reaction or an electrochemical reaction, such substances. Then the reaction products are transported separately to the surface where the products are subjected to a reverse (exothermic) reaction, and energy of this exothermic reaction is converted into electrical energy by the steam turbine and the combustion turbine or steam turbine, and combination of the combustion turbine. In some cases, a turbine (turbine) fuel element may be used.
The thermal reaction such as the endothermic reaction at the bottom of the well may proceed slowly at relatively low temperatures, the products are isolated in the trap, and a large area. The exothermic reaction will proceed rapidly and reach a high temperature, effectively concentrating the thus deep earth heat, making the production of electricity more efficient. In the first embodiment, a catalytic device having one or more channels, such as tubes or porous rods, for collecting one or more of the products of the endothermic reaction and transporting such product (s) separate from the other product (s). These channels are formed in the ceramic material, permeable products, wherein the ceramic material is surrounded by a thin film or mesh catalyst such as zeolite. Although the injected water automatically undergoes the endothermic reaction under the influence of heat downhole to catalytic use of the catalyst surface of the device is required for accelerating the reaction. Pipes or conduits have a cross sectional shape that provides a collection efficiency corresponding products for which they are intended.
One channel or set of channels made of a material which is permeable for one of the products of the endothermic reaction but is impermeable or repellent (for example, chemically, by higher pressure) the other product (s) and endothermic reaction reagent (reagents). Another channel or set of channels receives the remaining product (s). Pipes to be installed so as to accelerate the separation of the products by absorbing them separately as they form on the catalyst surface. In its simplest form, the catalytic device is a channel (containing the catalyst), which is permeable by only one of the endothermic products. Another product and remaining reactants, if any, will be removed from the borehole bottom through a separate channel.
In the first embodiment, the catalyst is permeable to products of the endothermic reaction. Selective (selects) a material which is permeable for only one product surrounds the tubes or porous conduits that are closest to the surface of the catalyst so that a product removed from the catalyst. The innermost tube (or porous conduit) picks up the remaining product. For example, if the desired endothermic reaction is the decomposition of water, the catalyst may be acceptable transition metal such as palladium. The catalyst material is a thin film or mesh surrounding the porous ceramic material in which channels are formed for the products. In a first preferred embodiment, the number of external channels absorb hydrogen, and an inner channel absorbs oxygen. The inner conduit may be a simple hole in the porous ceramic material through which oxygen diffuses. A number of channels exclusively for reception of hydrogen may be made, for example, palladium or other materials which are sufficiently porous to pass hydrogen, but not oxygen.
When the respective tubes absorb the respective products, the endothermic reaction is carried out using a catalyst will effectively decrease the total number of molecules on the outer side of the catalytic device. Since the porous catalytic device effectively removes the endothermic products from porous rocks, overpressure in the porous rock will not prevent the endothermic reaction. In fact, the increased pressure in the bottom hole accelerates an endothermic reaction. The optimum design of a particular catalytic device will depend on the nature of the endothermic reaction, its reactant (reactants) and products, the type of catalyst used and the conditions under which this reaction occurs.
Catalytic device according to the present invention will enable the endothermic reaction and simultaneously capture and separate the products of such reactions. The system according to the present invention preferably includes a mechanism for trapping products for the endothermic reaction to transport them to the wellhead. The apparatus of the present invention will catch the products and at the same time to separate them in order to prevent unwanted reactions between the products or product with some other material. The apparatus of the present invention will cause elevated pressures in the wellbore to accelerate the endothermic reaction. Higher pressures do not oppose the reaction since the porous conduits receive the reaction products.
In another embodiment, instead of using a catalytic device to catalyze the endothermic reaction, for the generation of the endothermic reaction can be used any of several reactions. A preferred endothermic reaction is the decomposition of water into hydrogen and oxygen. During the subsequent exothermic reaction is obtained clean water that can be pumped back into the well for another cycle. However, the temperature is usually needed for the thermal decomposition of water is not present in the earth's crust at a depth that is now practically can be achieved. Thus, the decomposition of water may be obtained by a sequence of reactions having sufficiently low activation energy (e.g., 4H 2 O + 2SO 2 + 2I 2 ---> 2H 2 SO 4 + 4HI and 2H 2 SO 4 ---> 2SO 2 + 2H 2 O + O 2 and 4HI ---> 2I 2 + 2H 2 which generally provide reaction 2H 2 O ---> O 2 + 2H 2) to allow the decomposition of water under the conditions obtained in the well. Thereafter, the decomposition products are collected and transported separately to the surface where they can be stored (separately) until used in the exothermic reaction. The product of the exothermic reaction is then returned to the well in the closed loop.
Another reaction that can be used is the reaction of "water gas", for example, the reaction of CH 4 + H 2 O ---> CO + 3H 2 which occurs spontaneously at a temperature of 800 o C. However, the exothermic reaction is complete, most of such reactions It may require oxygen from the atmosphere, and (they require oxygen or less) are during the subsequent exothermic reaction may produce carbon dioxide, nitrogen oxides and some other undesirable products. Also, efficiency of the device may be lost due to the need to use heat exchangers or other means to handle certain reaction products.
A second embodiment of the present invention is a system (for efficient electricity generation) with a thermocouple in which a thermocouple junction is located in the well at a depth at which geothermal heat is sufficient to create a temperature difference relative to the other thermocouple junction temperature. The temperature difference will cause the thermocouple generate electricity. In the simplest embodiment, one juncture of the thermocouple is located in the well, the other junction is set at a relatively low temperature outside of the well at the surface, and the resulting electricity is supplied directly to the purchaser or consumer of electricity.
In another embodiment, one juncture of the thermocouple is located in the well, the other junction is set at a relatively low temperature outside of the well at the surface, and the resulting energy is used to dissociate the chemical compound (e.g., water) to the endothermic products (e.g., hydrogen and oxygen) by electrolysis. Such electrolysis can be carried out in a well, in which case the products are transported through a pipeline to the surface, or the electrolysis may be conducted outside of the well at the surface. After that the products (e.g. hydrogen and oxygen) is an endothermic reaction is used as fuel, as described above, to generate electricity.
In a second preferred embodiment, a thermocouple is used together with the channels described above but without the catalyst. One junction of the thermocouple located in a well on the outside channels and one - within the channels. The first junction is located on the outer side of the channel more susceptible to the geothermal heat than the second juncture. Junction located within the channel, is colder than the juncture outside the conduit, as the pressure inside the conduit is much lower than the pressure outside the channel, causing the channel that is set within a low temperature. Since the second junction located within the channel is at a lower temperature than the juncture outside the conduit, the thermocouple due to the temperature difference will generate electricity. This energy is used to decompose the chemical compound (e.g., water) to the endothermic products (such as oxygen and hydrogen) by electrolysis, after which they are transported by pipelines up from the well and is used as fuel for power generation, as described above. However, it is obvious that within the scope of the present invention are acceptable and other reactions to produce an exothermic reaction reagents carried to generate electricity.
Systems generating electricity according to the present invention possess certain advantages over known power generating systems. The major advantage over prior art geothermal systems is that the art that the system according to the present invention by the endothermic reaction absorbs more heat per unit volume than can be captured by the heated brine or steam. For example, by decomposition of a given mass of water trapped in the five to six times more heat than it can be done by the same mass of steam. more efficient electricity production - Further, higher temperatures and therefore can be attained by the exothermic reaction.
Furthermore, since the present invention does not require a saline solution, the use of geothermal energy to generate electricity in accordance with the present invention is not limited to places laying wells having porous economically valuable underground rocks containing hot brine solution. Furthermore, efficiency is lost due to the need to use heat exchangers to prevent the precipitation of minerals in the generating device. Because the endothermic reaction products are contained separately, the energy absorbed in the bottom hole is not lost during transport to the surface energy. Such reaction products are corrosive to the equipment. No toxic gases that might be released into the atmosphere. endothermic reaction products transfer energy to a much lesser extent, and for this reason the well bore to be drilled has a much smaller diameter and is thus less expensive to manufacture. Moreover, instead of two only one well is required, since the injected water will not react chemically with the endothermic reaction products that are removed from the well over separate pipelines. Water injection will be carried out in the "operating" well. The result will be saved a lot of energy expended in pumping by the pump, currently used for the forced supply of water from the injection well through the fractures, loss of water in the rock will be lower, and well performance should be increased, as shown by testing the existing geothermal operational wells.
Furthermore, no increase will occur and mineral deposition related problems in the well. It will not take reinjection or disposal of brine. In a sense, the endothermic reaction is (in general) the decomposition of water is not released pollutants released into the atmosphere, and no useless expense of limited resources. endothermic reaction products may be stored and used when there is a need for electricity. If the products of the endothermic reaction come out of the ground at high pressure, they can be stored and used at high pressure, eliminating the need to compress them prior to the exothermic reaction (the operation that requires significant energy in combustion turbines) or if the exothermic reaction does not require compression, the overpressure of wells can be used to generate additional energy.
A preferred apparatus for carrying out an exothermic reaction is a combination of the internal combustion turbine in which the fuel is supplied in the form of two or more reagents which interact in the exothermic reaction (the product (s) which may be condensed), and the refrigerator. In a preferred embodiment, such agents are hydrogen and oxygen which are obtained in the process of endothermic reaction provided downhole. The hydrogen acts as a fuel and, when mixed with oxygen burns to form water vapor. After the final power stage in which the internal combustion turbine by implementing an exothermic reaction product (s) of the exothermic reaction is condensed, thus reducing the counterpressure acting on the combustion turbine and increasing its efficiency. The preferred combination turbine will deliver fuel in the form of hydrogen and oxygen to be burned to produce steam and be condensed at the downstream end of the turbine. Such a combination turbine could be used as part of a system according to the present invention or to operate independently on other fuel sources. In an alternative embodiment, the system according to the present invention can be used, and either a standard combustion turbine or a boiler combined with a steam turbine or fuel element.
Combination turbines, according to the present invention have several advantages. Through condensation product (Product) of the exothermic reaction, the combination turbine will reduce the back pressure of exhaust gases from the combustion turbine and increase the pressure drop across the final stages of the internal combustion turbine. Preferably, a powerful combination turbine section usually had more power stages than powerful internal combustion section prior art turbines, thus providing a greater share of the energy of the exothermic reaction, increasing the efficiency of the turbine and simultaneously simplify the condensation of steam at the outlet of the turbine. In addition, the combination turbine will not require a heat exchanger to produce steam, increasing accordingly its effectiveness. In a sense, the condensation provides a "closed-loop" (ie, all the products are condensed or captured in any other way), where you can make productive use of any energy that is otherwise lost in the exhaust gases, and further increase efficiency. The combination turbine does not emit pollutants. Furthermore, if the combination turbine completely provide fuel from the captured sources (as in the preferred embodiment, which utilizes hydrogen and oxygen), the prevent the ingress therein of dirt and other impurities, in most internal combustion prior art turbines (causing wear and the need for regular cleaning operations) and saves energy, which in the combustion turbine of the prior art used for condensation, filtration, and for heating or cooling supply air. Furthermore, unlike systems in which to generate electricity using solar energy or water, the combination turbine of the present invention can (depending on the reagent storage capacity) operate on demand as the peak of the unit or as a unit with the base load.
BRIEF DESCRIPTION OF THE DRAWINGS
Below is a detailed description of the preferred embodiments of the present invention, explaining these symptoms. These embodiments illustrate the new and unobvious system for geothermal power generation, according to the present invention, with reference to the accompanying drawings, which are given for illustrative purposes only. In these accompanying drawings like reference numerals indicate like elements.
 |
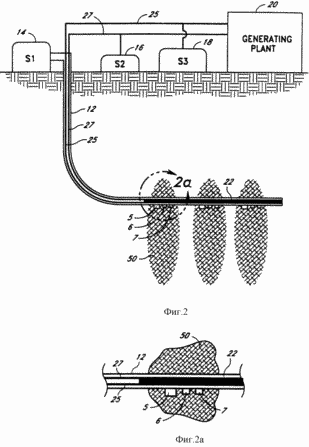 |
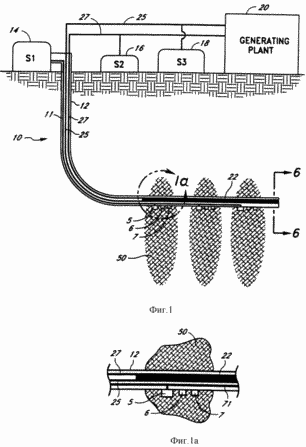
FIG. 1 - schematic section endothermic system of the preferred embodiment of the present invention.
FIG. 1a - bottom section enlarged view of the well system of FIG. 1.
FIG. 2 - schematic section of another preferred embodiment of the system of the present invention illustrating an alternate means of removing water in the hot dry rock.
FIG. 2a - cut face magnified image wellbore system of FIG. 2.
 |
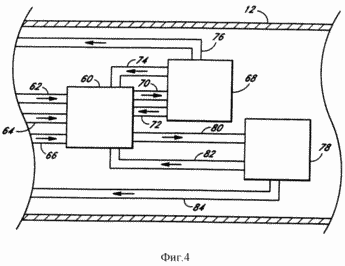 |
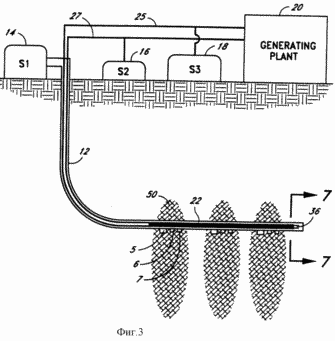
FIG. 3 - enlarged schematic downhole additional embodiment of a system according to the present invention.
FIG. 4 - schematic enlarged downhole section of another embodiment of the system according to the present invention.
 |
 |
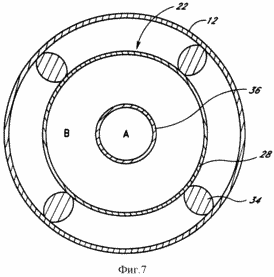 |
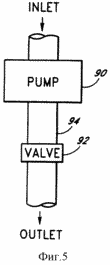
FIG. 5 - schematic representation of an enlarged section of the pipe, shown by way of example, the compound used in the camera illustrated in FIG. 4.
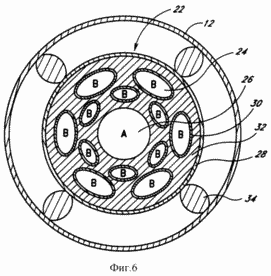
FIG. 6 - an enlarged cross sectional view taken along line 6-6 of FIG. 1, illustrating elements of the catalytic device of the system.
FIG. 7 - an enlarged cross sectional view taken along line 7-7 of FIG. 3, illustrating an alternative embodiment of the catalytic device of the system.
 |
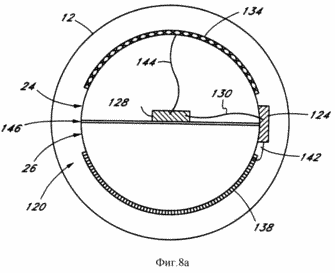 |
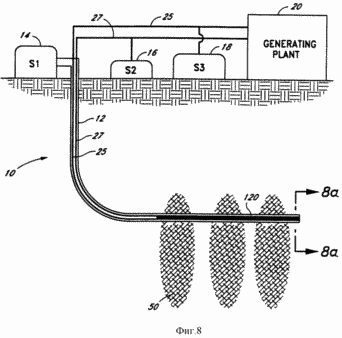
FIG. 8 - a schematic sectional view of a preferred embodiment of the electrolysis system of the present invention.
FIG. 8a - enlarged cross-section bottom hole system shown in FIG. 8.
 |
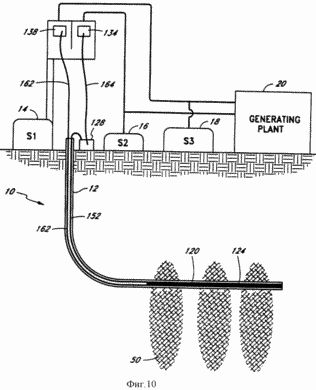 |
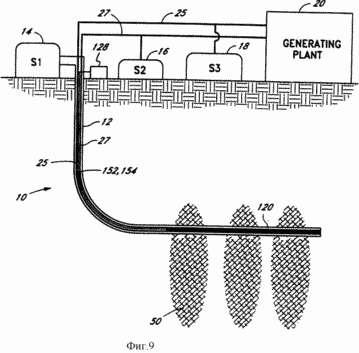
FIG. 9 - a schematic representation of the electrolytic cutting system of another embodiment according to the present invention.
FIG. 10 - a schematic sectional view of the electrolysis system of another embodiment according to the present invention.
 |
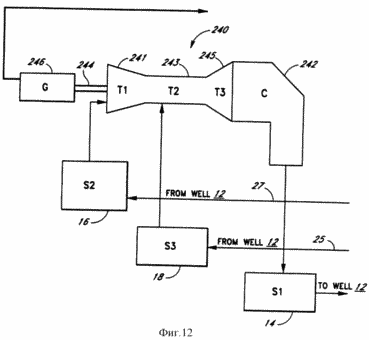 |
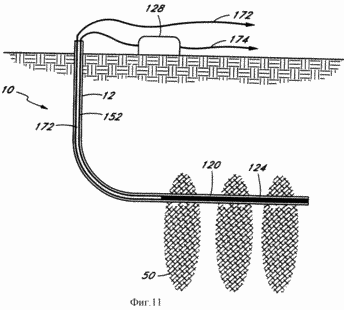
FIG. 11 - schematic representation of an electrolytic cutting system further embodiment of the present invention.
FIG. 12 - a schematic representation of the combined turbine used in the system according to the present invention.
Inscriptions to the drawings
|
By FIG. 1, 2, 3, 8, 9 and 10: 1 - Power |
By FIG. 5: 1 - Entrance |
By FIG. 12: 1 - 12 from the wells |
DETAILED DESCRIPTION OF THE DRAWINGS
The present invention provides systems and methods for capture and use geothermal heat using a thermal process. Within preferred thermal process produces products that are exothermic reactants. Then, as a result of the exothermic reaction products of the thermal process can generate electricity. In this application, two preferred thermal process is described.
GEOTHERMAL POWER GENERATION SYSTEM of catalytic DEVICE
FIG. 1 illustrates the geothermal generating system 10 of the present invention. With the system 10 of the present invention for converting geothermal heat to electricity may be used effectively formation viable hot, dry rock. The system 10 of the present invention eliminates the problems of environmental pollution, less expensive and has a much higher productivity. The system 10 comprises a well 12 coupled to the storage tank 14, indicated in FIG. 1, symbol S1, for storing the reactants that are to be used for the endothermic reaction which takes place in the bottom of the well 12 in fracture zone 50 of hot, dry rock. It is assumed that the system 10 of the present invention may be used elsewhere in the earth's crust, such as in porous rocks where deep Earth heat enough to activate the desired endothermic reaction. The catalytic device 22, which catalyzes the desired endothermic reaction, it is established in the bottom section of the well 12, and the porous conduits or chambers 24 and 26 (shown in FIG. 6) of the catalytic device 22 coupled to standard conduits 25 and 27, respectively, which extend upwardly through the well 12. the standard conduits 25 and 27 transport the products of the endothermic reaction 12 downhole to the surface, where the products can be stored in storage tanks 18 (S3) and 16 (S2), respectively, or be fed directly to the plant 20 for conversion to electricity . endothermic reaction products in accordance with the present invention separately transported through porous channels 24 and 26 and then through the conduits 25 and 27 to the combination turbine of the present invention. In one embodiment, the energy of these products released during the exothermic reaction as will be described in more detail below. In turn, this energy is converted into electricity.
In a preferred embodiment, a compound or chemical reagent stored in storage tank 14 is water, which bottom hole 12 is decomposed into hydrogen and oxygen. Storage tank 14 supports the well 12, a column of water. Because high pressure ambient downhole environment 12 generated by the water column in the well 12, the increased pressure causes the endothermic reaction products to pass through the catalytic device 22 in the porous conduits or chambers 24 and 26 and upwards through the conduits 25 and 27.
Separate conduit 11 connected to the storage tank 14 and extends downwardly to the bottom of the well 12, where the water from the water conduit 11 may be released from the hole 12 in the fracture zone 50 through a one-way gidroraspredeletel 5 in well 12. To create a fracture zone 50 in the hot dry mountain rock cracks to expand and access larger rock volume for the circulation of the medium pumped water. As part of the water is lost in the cracks of the rock, must from time to time to replenish the water through the fracture zone way valve 5. In the preferred embodiment, water is pumped into the fracture zone 50 is fed from a separate conduit 11, instead of the water column in the well 12, as water pumped into the fracture zone 50, it is easier to adjust from the conduit 11, than the water in the well 12. The pressure gauge 6 and thermometer 7 on the outer part of the hole 12, as shown in FIG. 1 and FIG. 1a, pressure is measured and the temperature of the fracture zone 50 so as to notify the operator when to inject a fault zone 50 more water. In that case conduit 11 to actuate the movement of water is connected to a pump (not shown) on the surface.
In accordance with FIG. 1 endothermic reaction takes place in the horizontal wellbore section 12, which is surrounded by the fracture zones 50. Instead of creating a horizontal section of the well may be drilled at an angle down (not shown). Heat generated from the fracture zones 50, raises the temperature of the casing 12, which accordingly increases the temperature of the water in the well 12. In this environment, the catalytic device 22 provides the possibility of initiating the endothermic reaction and the endothermic reaction products separation.
Instead of using one continuous section, as shown in FIG. 1, catalytic device 22 may be divided into a plurality of serially connected sections which are connected together by means of a relatively flexible tube (not shown). This arrangement is preferred because the flexible tube, such as standard tubing, will be cheaper than the continuous catalytic device section 22, which is essentially made of ceramics. Flexibility and preferred due to the need for directional drilling to access areas 50 fault. For connecting flexible nozzle tubes can be used to each catalytic device section (not shown), the pipe will be located in areas where there is no fault zone 50. Flexible piping, such pipe must be impermeable to the endothermic products and able to withstand temperatures up to 800 o C.
FIG. 6 shows a cross section of the well bottom 12 to provide a more detailed illustration of a preferred embodiment of the catalytic device 22. The catalytic device 22 is supported in borehole 12 by a plurality of rods 34, to give
possibility endothermic reactants to circulate around the catalytic device 22. The rods 34 may be protrusions or any other support device as is obvious to the skilled skilled the art. As shown in FIG. 6, the catalytic device 22 comprises porous ceramic material 32 having a porous conduit 26 disposed substantially at the center of the ceramic material 32. The ceramic material 32 is selected such that it has a structure which would be relatively permeable to the endothermic products, but at the same it's time to not activate the conversion reagent in the ceramic material 32.
The porous conduit 26 in the ceramic material 32 is substantially surrounded by a number porous conduits 24. The porous conduits 24 and 26 may be pipes or channels, and have a circular cross section, or other configuration, which is more efficient for collecting reaction products. The porous conduit 26 may be limited to a hole substantially in the middle of the ceramic material. The porous conduit 24 is made of a material which is porous (permeable) only on one of the endothermic products. In a preferred embodiment, in which the decomposition of water provide the porous conduit 24 is made from a suitable transition metal such as palladium, which is porous (permeable) for hydrogen, but not oxygen. The porous conduit 26 is marked in FIG. 6 by the letter A, which indicates that the porous conduit 26 receives endothermic product A, and the porous conduits 24 are marked with the letter B, which indicates that the porous conduits 24 receive endothermic product B. In a preferred embodiment, the product A method may include, for example, oxygen, and the product B - to hydrogen.
To accelerate the series of reactions to produce hydrogen and oxygen in the bottom of the well 12 on the catalytic device 22 is provided a thin film or mesh catalyst 28. Thus, the water in the bottom hole 12 reacts with the catalyst 28 on the surface of the catalytic device 22. Ceramic material is designed to be permeable to the endothermic reaction products so that the products have diffused into respective porous conduits 24 and 26. the porous conduits 24 and 26 are formed in the ceramic material 32 in order to accelerate the separation of the products by absorbing them as they form on the catalyst 28.
As shown in FIG. 6, each porous conduit 24 is made from a selective material 30 which tends to be porous (permeable) only with respect to product B. Thus, product B of the endothermic reaction flows through the ceramic material 32 and is collected next to the porous conduits 24 after product B diffuses through the selective (selecting) the material 30. Since the selective material 30 is specifically designed to block the passage of product A, as product A diffuses through the ceramic material 32, product A maneuvers around the locations of the selective material 30 and through the passages between a series of porous conduits 24 until until no product a diffuses into the porous conduit 26. As a result, the products a and B of the endothermic reaction are separated, acting in the corresponding channels 26 and 24. Some of product B may actually diffuse through the porous conduits 24 and actually pass into the porous conduit 26 where this amount of product B reacts with product A. this reaction does not appear to have any significant adverse effect on the system. For example, in the case of decomposition of water porous conduit 26 is filled with oxygen and a small amount of water vapor that can be dehydrated to the surface of the oxygen.
Another embodiment of the system 10 of the present invention illustrated in FIG. 3, where use different catalytic device 22. Contrary to the embodiment illustrated in FIG. 3, the horizontal section of the well 12 may be directed downwards at an angle (not shown). As shown in FIG. 3, the catalytic device 22 has a sleeve 36 with an open end facing the end of the catalytic device 22. Sleeve 36 extends from an open end through the catalytic device 22 and is coupled to a standard conduit 27, desirably through a nozzle (not shown). This embodiment of the present invention is illustrated in detail in the schematic cross section shown in FIG. 7. Just as in the case of the embodiment shown in FIG. 6, the catalytic device 22 is supported in the middle of the well 12 by a plurality of support rods or projections 34. The catalytic device 22 comprises a hollow channel formed from catalyst 28, pipe 36 and extending substantially in the center of the catalyst 28.
Preferably, the decomposition of water, the catalyst 28 is made from palladium which absorbs hydrogen into the hollow channel. Oxygen is not able to diffuse through the palladium tube and continues to slowly pass the end of the well where the oxygen actually enters the open end of the elongated tube 36 as will include water, ozone and hydrogen peroxide. Oxygen, ozone and hydrogen peroxide will be easier to seek the end of the well 12 when the horizontal section of the well 12 illustrated in FIG. 3, inclined downward. Oxygen, water, hydrogen peroxide and ozone through elongated tube 36, and then the standard conduit 27 is pumped back up to the surface. Before submission to the turbine for the exothermic reaction of oxygen and ozone are separated and the hydrogen peroxide can be separated from the mixture. Such separation can be accomplished by conventional means well known to those skilled in the art. The hydrogen which diffuses through the palladium catalyst 28, rises to the surface through the hollow portion of the catalyst 28 and then the standard conduit 25 due to high pressure in the bottom hole 12.
FIG. 3 shows that the catalytic device 22 performs two main functions: it provides the separation of the products and endothermic reaction, and it removes the products from the porous rock, so that increased pressure in the porous rock is not hindered conducting endothermic reactions. May catalyze the endothermic reaction more substances. However, the reaction products tend to readily recombine into the reactant (reagent) under the conditions that exist in the well. Moreover, the products of the endothermic reaction may be sufficiently reactive, especially at elevated temperatures, a chemical interaction with the walls of the well or otherwise react in an undesirable manner once they leave the surface of the catalyst. For this reason, the reaction products must be separated and trapped. Furthermore, when the endothermic reaction provides more moles of product than it consumes moles of reactant, the reaction will be inhibited highest ambient pressure that exists in the well 12. During operation the well 12, a column of water will create a very high pressure at the bottom of the water column. Because every 10 m were added 1-pressure atmosphere, the well was drilled to a depth of three kilometers, will downhole pressure of 12 to 300 atmospheres. Opposition pressure is a major obstacle to the reaction at the bottom of the well, which is at a considerable depth and at an elevated temperature will cause the pressure to increase significantly. However, since the channels or chambers 24 and 26 are permeable to the endothermic products, a very high pressure will force the products pass through the respective channels 24 and 26 and thereby effectively decrease the number of molecules on the outer surface of the catalytic device 22. Thus, increased pressure in the bottomhole well 12 accelerates an endothermic reaction.
In addition, increased pressure in the bottom hole 12 causes the endothermic reaction products to rise to the surface through the porous conduits 24 and 26 and then through the conduits 25 and 27. Thus, for transporting the reaction products to power the pumps 20 is not required, although devices such as pumps can be used.
FIG. 4 illustrates another means for activating the endothermic downhole 12. Since the temperature which is usually required for decomposing the water, not normally reached in the earth's crust at a depth that can currently be achieved by practical means, the system 10 illustrated in FIG. 4 does not directly decompose water into hydrogen and oxygen. Instead, the system shown in FIG. 4, carries out the decomposition of water as a result of a sequence of endothermic reactions having sufficiently low activation energy levels required to obtain the reaction products. Depending upon the conditions (primarily temperature and pressure) existing at the point of the endothermic reaction may be used any of several reactions.
In one such sequence of reactions used in the first reaction as the reaction
2H 2 O + SO 2 + I 2 ---> H 2 SO 4 + 2HI,
and the products of this first reaction is then decomposed in separate reaction chambers as follows:
2H 2 SO 4 ---> 2SO, 2 + 2H 2 O + O 2 and
2HI --- I 2 + H 2.
Thus, the overall endothermic reaction requires not only water, but sulfur dioxide and iodine. For this reason, in this embodiment, the bottom of the well 12 to the first reaction chamber 60 through individual pipes 62, 64 and 68, respectively, is transported by water, sulfur dioxide and iodine.
The first reaction chamber 60 was prepared an acidic sulphate salt, which is transported via conduit 70 to the second reaction chamber 68 where the hydrogen sulfate salt in water destroys, sulfur dioxide and oxygen. The water and sulfur dioxide is recycled back to the first reaction chamber 60 through pipes 74 and 72, respectively. The resulting oxygen from the second reaction chamber 68 is transported back up to the surface through a pipe 76. The first reaction chamber 60 and hydrogen iodide is obtained, which is transported via conduit 80 to a third reaction chamber 78, wherein hydrogen iodide is decomposed into hydrogen and iodine. The iodine is recycled back to the first reaction chamber 60 through the pipe 82 and hydrogen is transported to the surface through a pipe 84. The rate of the reaction sequence can be controlled (not shown) by means of valves in pipes feeding different chemicals to the respective reaction chambers, and valves controlled from the surface. Although oxygen and hydrogen are the only end products that are transported to the surface, the remaining end products, water, sulfur dioxide, and iodine are reactants are continuously consumed in the course of successive reactions and again fed into the first reaction chamber 60 to produce hydrogen and oxygen. Although as a result of the first reaction are sulfuric acid, this acid is immediately decomposed in the subsequent reaction. Moreover, because the reactions which take place in the second reaction chamber 68 and the third reaction chamber 78 require a very high temperature, the second and third reaction chambers 68, 78 should be located in sections of the well 12, which are in the fault zone 50.
FIG. 5 shows a further illustration of the transport mechanism of the chemical compound from one reaction chamber to another. FIG. 5 shows the pump 90 and valve 92 associated with the transport pipe 94 where the pump 90 and valve 92 are used for example for controlling gas flow within, for example, the transport tube 94, corresponding to the reaction chamber. Although the pump has been shown, it is shown as an example only, and depending on the various pressures used may require pumps (not shown) to facilitate gas transport. through pipes 76 and pumps 84 are not necessary for transporting the gaseous oxygen and hydrogen since elevated downhole pressure 12 should cause the oxygen and hydrogen to rise to the surface.
Another reaction that can be used is the reaction of "water gas"
CH 4 + H 2 O ---> CO + 3H 2.
which occurs spontaneously at a temperature of 800 o C. However, most such reactions may require oxygen from the air to complete the exothermic reaction and (they require no or air) during the subsequent exothermic reaction may produce carbon dioxide, nitrogen oxides and some other undesirable products. In addition, efficiency may be lost due to the need to use heat exchangers or other means to handle certain reaction products.
The main advantage of endothermic reactions in the system 10 of the present invention is that the system 10 by the endothermic reaction absorbs more heat per unit volume than can be captured by the heated brine or steam compared to reactions in geothermal systems of the prior art. For example, decomposition of a given mass of water trapped in the five to six times more heat than the same mass of steam. Due to the greater concentration of heat in the system of the present invention is obtained at higher temperatures, which improves the efficiency of the exothermic reaction in the turbine and the subsequent generation of electricity.
Furthermore, the present invention requires only one well in contrast to prior art systems that require two wells. Endothermic reactants may be transported in the same well as the endothermic products since there is no danger that the reactants and products will interact. This contrasts with systems of the prior art in which the injected water could not be transported in the same well as the rising steam, since steam will give (i.e. waste) heat water, decreasing accordingly the efficiency of the prior art systems . In addition, one well is used in the system of the present invention is less expensive to drill since the products of the endothermic reaction energy are in relatively much smaller volume than the steam or brine used in geothermal systems of the prior art. For example, in prior art systems to capture steam or brine from a reservoir cross-sectional area of the production well may be 36 inches (914.4 mm). Since the system 10 of the present invention requires approximately one-sixth the space, the cross-sectional area of the well system 10 of the present invention may require, for instance, only 12 inches (304.8 mm), of which 6 inches (152.4 mm) necessary for water injection, and 6 inches (152.4 mm) - for the transport of hydrogen and oxygen.
GEOTHERMAL POWER GENERATION SYSTEM Thermocouples
FIG. 8 illustrates another embodiment of the geothermal generating system 10 of the present invention. The well 12 is substantially similar to that shown in FIG. 1, except that the catalytic device 22 is replaced with a device connected to the conduits 25, 27 and containing a thermocouple 120. The part of the well 12 containing the thermocouple or electrolytic device 120 may be horizontal or downwardly extending at an angle (not shown). Pipes 25 and 27 are connected with porous channels or chambers 24 and 26 inside the thermocouple 120. The conduits 24, 26 supported in the wellbore by a plurality of rods or protrusions (not shown) to circulate around the outside of possibilities 24, 26 channels.
The electrochemical device 120 generates an electric current that can be used to generate electricity or to produce products of electrolysis that can be stored and used to generate electricity. Thus, the electrochemical device 120 is a device for converting thermal energy in the well 12 into electrical energy. In a preferred embodiment, the electrochemical device 120 is a thermocouple 120 which is mounted in the lower section of the well 12, and one junction 124 (hot junction) is located on the outside of the porous conduits 24, 26 used for transporting product, and therefore are at a higher temperature than the other juncture 128 (cold junction) of the thermocouple 120 which is positioned within one of the channels 24, 26. FIG. 8a shows the juncture 128 positioned within the channel 24. These two junctions 124, 128 are interconnected by means of the current carrying bus or 130. The resulting electrical current is supplied to two separate areas the surface channels 24, 26, creating an anode 134 (conduit 24), in which during electrolysis (electrochemical reaction) of the products obtained (e.g., hydrogen), and a cathode 138 (conduit 26), which is obtained by electrolysis other product of the electrochemical reaction (e.g., oxygen). Reagent (reagents) the electrochemical reaction (chemical compound amenable to electrolysis) stored in the storage tank 14 and supplied from the wellhead 12 to the thermocouple 120. An example of the electrolysis of the chemical compound amenable to electrolysis, is the decomposition of water into hydrogen and oxygen, which are the products of electrolysis. Obviously, for the conversion of thermal energy into electric other types of electrochemical devices can be used.
Junctions 124 and 128 of the thermocouple 120 are respectively connected to the anode 134 and cathode 132 via a bus or current-carrying means 142 and 144. The channel 24 containing the anode 134, preferably made of a material which is permeable electrochemical reaction product obtained at the anode 134 (for example, palladium if the product is hydrogen) and the conduit 26 comprising the cathode 138, preferably made of a material which is permeable to the electrochemical reaction of the product obtained on the cathode. Channels 24 and 26 are preferably not permeable to the chemical compound undergoing electrolysis (e.g., water), so that when the formed product of the electrochemical reaction, increased pressure in the well bore 12 causes it to pass into the corresponding channel 24 or 26 to 24 or 26 at the channel surface. when the product passes into the channels 24 or 26, the pressure drop causes a drop in temperature of the product (in the channel 24 or 26), which cools the juncture 128 of the thermocouple 120 that is located in the channel 24 or 26. the pressure inside the channels 24 and 26 will nevertheless sufficiently high to push products to the wellhead 12.
The products of electrolysis are transported separately by a porous channels 24 and 26 and through the conduits 25, 27, for example, to storage tanks 18 and 16 or 20 to the power plant, to generate electricity. As in the above-described embodiments, the energy of the electrochemical reaction products released during the exothermic reaction and converted to electrical energy. The generating system 10 may be used water directly from the water circulating in the well 12.
Channels 24 and 26 have a semicircular cross section as shown in FIG. 8a, and form between them a wall 146 that is not permeable to the electrochemical reaction products. These two channels 24 and 26 together have a circular cross section in the well 12. The circular cross section of the aggregate of the two channels is advantageous to minimize the size of the well 12 that needs to be drilled. For this reason, the size of the hole 12, the channels 24 and 26 of semi-circular cross section occupy the maximum internal volume. This amount, in turn maximizes the pressure differential between the region inside the conduits 24, 26 and the area outside it. The differential pressure is desirable since it causes the corresponding product to pass into the channel 24 or 26, wherein the cooled junction 128 of the thermocouple 120 that is located in the channel 24 or 26. The pressure in the conduit 24 or 26 remains sufficiently high to drive the electrochemical reaction products by toward the earth's surface. Although FIG. 8a shows a double wall formed by the walls of the two channels 24 and 26, it is obvious that a single wall that is impermeable to both products can also be used instead of the double wall.
However, the channels 24, 26 apart semicircular may have any cross-sectional shape. For example, channels 24, 26 may be circular (not shown). The internal volume of the channels 24, 26 will be half of the volume occupied by channels having a semicircular cross-sectional shape of the embodiment shown in FIG. 8a. Thus, the pressure difference between the region inside the conduits 24, 26 and their area is to be less than the pressure drop in the embodiment with the channels 24, 26 having a semicircular cross-sectional shape.
FIG. 9 illustrates another embodiment of the system 10 of the present invention. In this embodiment, the system 10 does not depend on the temperature inside the lower one of the conduits or chambers 24, 26 to cool one thermocouple junction 128 120. Instead, the juncture 128 is located on the surface of the well 12, where it is kept at a low temperature, and is connected by a two tires, a tire 152 - with the hot junction 124 of the thermocouple 120 located outside channels 24 and 26 in the bottom of the well 12, and another bus 154 - with the anode 134 on the surface of one of the channels 24 and 26 in the bottom of the well 12 (similar to the way shown in FIG. 8a). At the cathode 138 and the anode 134 during the electrolysis process will be allocated the appropriate electrochemical reaction products (e.g., hydrogen and oxygen), and these products would be captured.
FIG. 10 shows another embodiment of the system 10 of the present invention. In this embodiment, the thermocouple junction 120 being the hot junction 124 (similar to that shown in FIG. 8a), located at the bottom of the well 12 and connected to bus 152 with junction 128 of the thermocouple 120 being the cold junction, which must be kept at a lower temperature and which is located on the surface of the well 12. Эти два спая 124, 128 соответственно соединены посредством шин 162 и 164 с катодом 138 и анодом 134 вне скважины 12 на поверхности, где улавливают продукты электролиза и используют их в качестве топлива для выработки электроэнергии. В этом альтернативном варианте скважина 12 не содержит каких-либо каналов.
FIG. 11 illustrates a further embodiment of the system 10 of the present invention. In this embodiment, the juncture 124 (similar spayu shown in FIG. 8a) of the thermocouple 120 that is to be kept at a high temperature, and is located at the bottom of the well 12 and connected to bus 152 with junction 128 of the thermocouple 120 that is to be kept at a low temperature and which the well 12 is located on the surface. Electricity generated by the thermocouple 120 is supplied through the wires 172 and 174 to the purchaser or consumer of electricity. In this case there is no need for reagents electrochemical reaction channels and the internal combustion turbine or other generating device described below. However, it should be noted that other thermal processes that can produce reactants such as the exothermic reactants for power generation reaction is known to those skilled in the art in the art and are within the scope of the present invention.
combination turbine
FIG. 12 illustrates a schematic diagram of a combined turbine 240 in which an exothermic reaction is carried out for the release of geothermal heat. The combination turbine 240 comprises a turbine compressor stage 241, stage 243 of the fuel injector and the combustion chamber of the turbine, the turbine power stage 245 and a fridge 242. Steps 241, 243 and 245 and the refrigerator 242 preferably have a structure which is known to those skilled in the art. Combined turbine generator 240 via a shaft 244 connected to generator 246, in which the mechanical energy of the rotation shaft 244 is converted into electric power generator.
Turbine compressor stage 241 receives exothermic reactant A, which is the product A of the endothermic (or electrolytic) reaction, from the storage tank 16 or directly from the well 12 through the conduit 27 (FIG. 1). Depending on the type of exothermic reactant A (endothermic or A product of the electrochemical reaction) Reagent A does not necessarily need to be compressed and thus the compressor stage 241 may not be required. In the preferred embodiment, exothermic reactant A is oxygen. Since the oxygen coming from the well 12 is already under pressure, due to pressure in the well 12, the oxygen must be compressed sufficiently to need passage 241 of the compressor stage. Step 243 and the fuel nozzle turbine combustor receives exothermic reactant B, which is the product B of the endothermic (or electrolytic) reaction, from the storage tank 18 (or directly from the well 12) through conduit 25 (FIG. 1). In the preferred embodiment, exothermic reactant B is hydrogen.
In step 243 exothermic reactant B, i.e., hydrogen, acts as a fuel and combusted when mixed with exothermic reactant A, i.e. oxygen, forming a large amount of heat and steam. The resulting energy released by the exothermic reaction is used to rotate the blades (turbine) in the power stage 245 which in turn rotates the generator shaft 244. After the exothermic product (steam) passes through the turbine power stage 245, the exothermic reaction product is immediately condensed in the condenser 242 where the exothermic vapor product is converted into liquid. Efficiency improves turbine 240 through the exothermic condensation reaction product to remove the back pressure from the turbine 240. The condensation product of the exothermic reaction can be performed by means known to those skilled in the art. In a preferred embodiment, the water vapor is condensed (converted) into the water, which is fed into the storage tank 14 to the reagent endothermic (electrolytic) reaction, for re-injection into the well 12.
By combining the combined turbine condenser 242 to 240 obtained higher efficiency turbine combined according to the present invention than in combustion turbines of the prior art, a steam turbine and when used together with a combustion turbine and condenser. In a preferred embodiment, the efficiency increases because the combination turbine does not require a heat exchanger to convert the water vapor into the product of the exothermic reaction heat. In prior art systems the combination turbine of the present invention can not be used because the exothermic product is not susceptible to condensation pollutant as opposed to giving in water vapor condensation, obtainable in the combination turbine 240 of the present invention.
Furthermore, it allows condensation to obtain a closed system in which all the exothermic product is condensed or otherwise captured, and it becomes possible productive energy (which in prior art systems with the exhaust gases is lost) further, thus increasing efficiency. In contrast to prior art systems combined turbine 240 according to the present invention does not give off pollutants to the atmosphere. In addition, because the combination turbine 240 in the preferred embodiment hydrogen is used as fuel and oxygen, which are controlled by springs, to prevent the ingress of dirt and other impurities that are in most combustion turbines can enter from the atmosphere. Since the present invention relates to the products of the endothermic (or electrolytic) reaction for conveying the geothermal heat, in contrast to prior art systems in which the extracted steam or brine has to be used immediately, these products can be stored for later use after a while. Accordingly, the combination turbine 240 of the present invention has the added flexibility of operating as a peak of the unit on and off on demand, or as a baseload unit which operates at a constant speed.
Alternatively, the system 10 of the present invention may be used with a conventional combustion turbine, or a boiler with a steam turbine, and the products of the endothermic (or electrolytic) reaction might be used in a fuel cell.
Furthermore, it appears that the products of the endothermic (or electrolytic) reaction, for example, hydrogen and oxygen, are useful by themselves and the present invention can be used to obtain these products and store them at the surface of the well 12 for their use other than to generate electricity .
In addition, the present invention can be used in environments other than the geothermal well 12 and is suitable for use in any environment, natural or man-made, having suitable temperature and pressure.
The above is a description of the best embodiment of the present invention as well and a method of embodiments and applications, when using such a comprehensive, clear and precise terms, can be understood by any person skilled in the art for which it is intended, with a view to its embodiments and applications. However, the present invention may be modified and embodied in the form of constructions which differ from those described above, but it is completely equivalent. Therefore, the present invention is not limited to the specific embodiments thereof. On the contrary, the present invention encompasses all modifications and alternative constructions, corresponding to the volume and nature of the invention which are described in general terms in the claims below, in which, in particular, the articulated object to claim the invention.
CLAIM
1. A system for capturing geothermal heat and releasing the heat in the exothermic reactions to convert to electricity comprising a well having a top and a bottom, where said well is drilled to a sufficient depth to capture energy from geothermal heat when the reactants are introduced into said well to cause reactions of said reactants; device located in the bottom of said well, said device captures geothermal heat and for separating output products; first and second conduits for transporting said output products to the top of said well; and means coupled to said first and second conduits and the output for use products, to produce an exothermic reaction to generate electricity.
2. System according to Claim. 1, in which said bore is connected to a first storage reservoir for storing said initial reagents.
3. The system of claim 1, further comprising a second storage tank connected to said first conduit for storing a first output product.
4. The system of claim 1, further comprising a third storage tank is connected to said second conduit for storing a second output product.
5. The system of claim 1, wherein said means connected to said first and second conduits comprises a combustion turbine coupled to a condenser.
6. The system of claim 5, wherein said turbine further comprises a compressor connected to said turbine inlet.
7. The system of claim 5, wherein said output is connected to the refrigerator with the first storage tank for storing said reactants.
8. The system of claim 1, wherein the well is drilled to a fracture zone of hot, dry rock.
9. The system of claim 1, wherein the output products are endothermic products obtained by endothermic reactions.
10. The system of claim 9 wherein the endothermic reaction is the decomposition of water.
11. The system of claim 9, wherein the device is a catalytic device.
12. The system of claim 11, wherein said catalytic device comprises a catalyst that is permeable for both the first and second products for endothermic reactions, a first porous conduit to said catalyst for receiving said first product, a second porous conduit in said catalyst for receiving said second product, and a selective material surrounding said second porous conduit, said selective material is permeable only to said second product.
13. The system of claim 12, wherein said first porous conduit is coupled to said first conduit for transporting said first product to the top of said well, and said second porous conduit is coupled to the second conduit for transporting said second product to the top of said well.
14. The system of claim 11, wherein said catalytic device comprises a catalyst which is permeable only to the first product endothermic reactions, a first porous conduit to said catalyst for receiving said first product, at least one second porous conduit connected to said catalyst and for receiving a second product endothermic reactions, and a selective material surrounding said second porous conduit, said selective material is permeable only to said second product.
15. The system of claim 11, wherein said catalytic device comprises a catalyst which is permeable only to a first product of said endothermic reaction, and a return duct extending from the end of said catalytic device for extracting the remaining products of said endothermic reaction, said return duct impermeable for said first product.
16. The system of claim 1, wherein the output products are the products of the electrochemical reaction derived by electrochemical reactions.
17. The system of claim 16, wherein the electrochemical reaction is water decomposition reaction.
18. The system of claim. 16 wherein the device is an electrochemical device.
19. The system of claim 18, wherein the device is a thermocouple.
20. The system of claim 19, wherein said thermocouple comprises a first porous conduit for receiving a first product, a second porous conduit for receiving the second product and coupled to said first porous conduit, a selective material surrounding said second porous conduit, wherein said material is a selective permeable only to the second product, reference junction positioned within and coupled by a first bus to a surface of one of said first and second porous conduits, the hot junction located outside said porous conduits and connected through a second bus to the surface of the other one of said first and second porous channels, said cold junction and the hot junction of the specified connected via a third bus.
21. The system of claim 20, wherein the cold junction is connected via the first bus with the first surface of a porous anode to form a channel, and the hot junction is connected by a second bus to the porous surface of the second channel to form a cathode, said junctions provide the power supply to said first and second buses.
22. The system of claim 20, wherein the first and second porous conduits are semi-circular cross-section with planar wall portions that are joined together.
23. The system of claim 19, wherein said thermocouple comprises a first porous conduit when receiving a first product, a second porous conduit for receiving the second product and coupled to said first porous conduit which is permeable only to the first product, and the hot junction is located outside of the stated porous conduits, said hot junction having a first bus, which is connected with the surface of said second porous passage, and a second bus which is connected to the cold junction, located at the wellhead, said first porous conduit having a surface which is connected to the cold junction, located at the wellhead.
24. The system of claim 23, wherein said first porous conduit is coupled to the first conduit for transporting said first product to the top of said well, and said second porous conduit is coupled to the second conduit for transporting said second product to the top of said well.
25. The system of claim 19, wherein said thermocouple has a hot junction, having a first current-carrying means which is connected to the cathode, and the second current-carrying means which is connected to the cold junction, said cold juncture connected through a third current-carrying means to an anode, and said cathode, anode, and cold juncture disposed at the wellhead.
26. The system of claim 1, wherein said means connected to said first and second conduits comprises a fuel element.
27. A system for capturing geothermal heat using endothermic reactions and releasing the heat in the exothermic reactions, comprising a well having a top and a bottom drilled to a sufficient depth for capturing geothermal heat in the endothermic reactions when reactants are introduced into said borehole; a first chamber located at the bottom of said well for receiving a plurality of reactants, where said reactants provide a first product and obtaining a second product; a second chamber located at the bottom of said well for receiving said first product from said first chamber, said first product decomposes to ensure receipt of the third, fourth and fifth product, said third and fourth product is transported to said first chamber, and said fifth product is transported to the top of said well; and a third chamber located at the bottom of said well for receiving said second product from said first chamber, said second product decomposes to ensure receipt of the sixth and seventh product, said sixth product is transported to said first chamber and said seventh product is transported to the top of said well .
28. The system of claim 27, further comprising a turbine for receiving said fifth and seventh products to generate exothermic reactions for generating electricity.
29. The system of claim 28, wherein said fifth and seventh products are oxygen and hydrogen.
30. The system of claim. 27 wherein said turbine comprises a combustion turbine coupled to a condenser.
31. A catalytic device for endothermic reaction products, to activate the catalyst contains an endothermic reaction, said catalyst is permeable to at least one of the products of the endothermic reaction; first and second porous conduits that are in contact with the catalyst for production and separation of the first and second products of the endothermic reaction; and a selective material surrounding said second porous conduit, said selective material is permeable only to the second product.
32. The apparatus of claim. 31 wherein said first and second porous conduits are located in said catalyst.
33. The apparatus of claim 31, wherein said first porous conduit is located in said catalyst, and said second porous conduit is located on the perimeter of said catalyst.
34. Thermocouple intended for power generation by the geothermal heat from the well having a top and a bottom, and which uses electricity for the electrochemical process, comprising a first junction, contained at the first temperature and disposed in the bottom of said well; second juncture contained at a second temperature which is lower than said first temperature; current-carrying means, coupled to said first and second junctions; wherein said second juncture is located in the first channel in the bottom of said well, the current-carrying means comprises a bus connecting said first junction to the cathode, and the other bus connecting said second juncture to an anode, said anode and cathode provide the electrochemical process of this implementation.
35. The thermocouple of claim 34, wherein said anode is formed on a surface of said first channel.
36. The thermocouple of claim 34, wherein said cathode is formed on the surface of the second channel in the bottom of said well.
37. The thermocouple of claim 36, wherein said first and second channels have a semicircular cross-section with planar wall portions that are joined together.
38. The thermocouple of claim 37, wherein said first and second channels are porous and permeable to receive a first product and a second product of electrolysis, respectively, said first porous conduit is permeable only to the first product.
39. The thermocouple of claim 34, wherein said second juncture is located at the mouth of said well, and said current-carrying means comprises a first bus connecting said first junction to said second junction, the second bus connecting said first junction to the cathode, and the third bus, connecting said second juncture to an anode.
40. The thermocouple of claim 39, wherein said anode is formed on a surface of the first channel in the bottom of said well and said cathode is formed on the surface of the second channel in the bottom of said well.
41. The thermocouple of claim 39, wherein said anode and cathode are positioned at the mouth of said hole.
42. The combination turbine for use in a geothermal system (for power generation), wherein the geothermal heat produces first and second products at the downhole depth at which said geothermal heat is sufficient to cause an endothermic reaction, comprising a combustion turbine for separate administration of said first and second products from the bottom of said well, actuated by the energy liberated in the exothermic reaction between said first and second products; refrigerator and connected to said combustion turbine for condensing the product of said exothermic reaction to reduce the back pressure at the outlet of said combustion turbine.
43. The combination turbine of claim 42, wherein said internal combustion turbine comprises a compressor coupled to the inlet of said combustion turbine.
44. The combination turbine of claim. 42 wherein said products are accepted turbine, which converts hydrogen and oxygen into water vapor.
45. The combination turbine of claim 42, wherein said refrigerator converts said steam to liquid water and returns the liquid water to the system.
46. A method for capturing depth Earth heat for generating electricity, comprising administering a reagent into the well, wherein said well has a depth sufficient to capture geothermal heat through thermal reactions; implementation of a thermal reaction in said well using said reactant; products and returning said thermal reaction to the surface of said well, said products generate electricity during exothermic reactions.
47. The method of claim 46, wherein the products are endothermic products obtained by endothermic reactions.
48. The method of claim 46, wherein the products are products of the electrochemical process, obtained by electrolysis.
49. A system for capturing and use geothermal heat using endothermic reactions and releasing the heat of the exothermic reactions in the process for generating electricity, comprising a well having a top and a bottom, where said well is drilled to a sufficient depth for capturing geothermal heat sufficient to activate said endothermic reaction; catalytic device disposed in the bottom of said well, said catalytic device and intended for separating the products of said endothermic reactions; means for supplying water from the mouth of said well to said catalytic device; a first chamber in said catalytic device having walls which are substantially impermeable to a first product of said endothermic reaction and substantially impermeable to a second product of said endothermic reaction; a second chamber in said catalytic device having walls which are substantially impermeable to said second product of said endothermic reaction; first and second lines respectively connected to said first and second chambers for transporting the endothermic reaction products to the top of said well, the high pressure in said borehole is used for force feeding said products at the depth of said catalytic device through said catalytic device and said first and second conduit to the mouth of said well; and a combination turbine coupled to said first and second conduits for using said products of the endothermic reactions to generate exothermic reactions for generating electricity.
50. A system for capturing geothermal heat using endothermic reactions comprising a well having a top and a bottom, where said well is drilled to a sufficient depth to capture the earth geothermal heat through the endothermic reactions when applying reagents into said well; catalytic device disposed in the bottom of said well, said catalytic device and intended for separating the products of said endothermic reactions; conduit for transporting products of the endothermic reaction to the top of said well; and means connected to said conduit for use the endothermic reaction products.
51. A system for capturing and use geothermal heat using electrolysis and heat liberation during the exothermic reactions for generating electricity, comprising a well having a top and a bottom, where said well is drilled to a sufficient depth for capturing geothermal heat sufficient to activate said process electrolysis; a thermocouple, located at the bottom of said well, said thermocouple is used for receiving and separating said products of electrolysis; means for supplying water from the mouth of said well to said thermocouple device; a first chamber in said thermocouple having a wall which is substantially impermeable to said first product of electrolysis and substantially impermeable to a second product of said electrolysis; a second thermocouple in said chamber having a wall which is substantially impermeable to said second product of the electrolysis; first and second lines respectively connected to said first and second chambers for transporting said products of electrolysis to the top of said well, the high pressure in said borehole at the depth of said thermocouple is used for force feeding said products through said thermocouple and said first and second conduits to the top of said well; and a combination turbine coupled to said first and second conduits for using said products of electrolysis to generate said exothermic reactions for generating electricity.
52. A system for capturing geothermal heat using endothermic process comprising a well having a top and a bottom, where said well is drilled to a sufficient depth for capturing geothermal heat through an electrochemical process when applying to the face of said well at least one chemical compound amenable electrochemical degradation; thermocouple, is at least partially disposed in said well, said thermocouple is used for receiving and separating the products of electrolysis of said chemical compound amenable to electrochemical decomposition; at least one conduit for transporting products of the electrochemical process to the top of said well; and means, coupled to said conduit for using said products of the electrochemical process.
53. A system for capturing the deep Earth's heat to generate electricity, comprising a well having a top and a bottom, said well is drilled to a sufficient depth to capture geothermal heat; thermocouple, at least partially disposed in said well, said thermocouple is used to generate electricity from said geothermal heat; and means connected to said electrochemical device (thermocouple) for supplying electricity.
54. The system of claim 53, wherein said electrochemical device includes an anode and a cathode coupled for generating said electric power.
55. The system of claim 54, wherein said means includes electrical lines connected to the anode and the cathode for power transmission.
print version
Publication date 10.01.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.