| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2204183
![]()
HYDROGEN-OXYGEN (AIR) FUEL BATTERY ELEMENT-BATTERY
The name of the inventor: Karichev Ziya Ramizovich
The name of the patent owner: Karichev Ziya Ramizovich
Address for correspondence: 129626, Moscow, Kuchin per., 12, apt. 1, Z.R. Karicheva
Date of commencement of the patent: 2001.11.30
The invention relates to the field of electrical engineering, in particular to fuel cells (TEs) used in power plants of various purposes, for example, on vehicles. According to the invention, the hydrogen-oxygen (air) fuel cell-battery (VKTEA) contains a hydrogen gas diffusion electrode with a hydrogen chamber, an oxygen (air) gas diffusion electrode with an oxygen (air) chamber and an electrolyte located between the hydrogen and oxygen (air) electrodes. In this case, the hydrogen electrode contains a metal hydride alloy, an oxide-nickel electrode is located between the hydrogen and oxygen (air) electrodes, and an alkaline electrolyte is taken as the electrolyte. The oxide-nickel electrode can be separated from the hydrogen and oxygen (air) electrodes by separators. The oxide-nickel electrode may be configured to be electrically connected to the oxygen (air) electrode by means of a switch. The oxide-nickel electrode can have a porosity in the range of 60 to 80% with a relative resistance of 1.5 to 8 in the electrolyte. The metal hydride alloy is located on the surface of the hydrogen electrode facing the hydrogen chamber, and the charge capacity of the oxide-nickel electrode is matched to the charging capacity On hydrogen metal-alloy alloy. The technical result of the invention is the creation of VKTEA having high specific characteristics and operating life.
DESCRIPTION OF THE INVENTION
The invention relates to the field of electrical engineering, in particular to fuel cells (TEs) used in power plants of various purposes, for example, on vehicles.
Hydrogen-oxygen (air) fuel cells, used on vehicles as an energy source, are known. However, fuel cells can not provide the peak power consumed by the vehicle during maneuvers, and energy recovery during braking. To meet these requirements, an energy storage device is used, for example a battery connected in parallel with the FC (see US Pat. No. 5,929,994, cl. H 02 J 7/00, 1999). The presence of an additional battery degrades the specific characteristics of the installation.
A hydrogen-oxygen fuel cell (VKTEA) is known, which is a fuel cell combined in one housing with an electrolyzer. VKTEA contains a hydrogen gas diffusion electrode with a hydrogen chamber, an oxygen electrode with an oxygen chamber, an electrolyte located between the electrodes, and storage tanks for hydrogen and oxygen (see U.S. Patent No. 4,839,247, H01M 8/18, 1989). A disadvantage of the known VKTEA are low specific electrical characteristics due to the presence of storage tanks for reagents. In addition, this VKTEA has a limited resource due to the poor reversibility of the oxygen electrode during cycling (see VS Bagotsky et al., Chemical sources of current, Moscow: Energoizdat, 1981, p. 255).
VKTEA is known, containing hydrogen and oxygen chambers with corresponding electrodes separated by electrolyte. To ensure a long working life, VKTEA contains two types of electrodes, some of which are used for charging, and others for the discharge of VKTEA (see U.S. Patent No. 4,074,018, Cl. H01M 4/86, 1978). The disadvantage of the VKTEA in question is the complexity of the design, due to the presence of two types of electrodes placed in one housing.
Among the known VKTEA, VKTEA, which is the most similar in terms of the set of essential features and the technical result achieved, is VKTEA containing a hydrogen gas diffusion electrode with a hydrogen chamber, an air gas diffusion electrode with an air chamber, an electrolyte located between the hydrogen and air electrodes, and a hydrogen storage container filled with a metal hydride alloy See U.S. Patent No. 5,510,202, H 01 M 8/06, 1996). The use of air as an oxidizing agent and metal hydride as a hydrogen storage device makes it possible to substantially increase the specific characteristics of the VKTEA by eliminating the oxygen capacity and reducing the operating pressure in the hydrogen tank. The disadvantage of this known VKTEA is associated with low reversibility of the air electrode during cycling, which leads to a decrease in the resource.
The object of the invention is to provide a VKTEA having high specific characteristics and a service life.
This technical result is achieved by the fact that VKTEA contains a hydrogen gas diffusion electrode with a hydrogen chamber, an oxygen (air) gas diffusion electrode with an oxygen (air) chamber and an electrolyte located between the hydrogen and oxygen (air) electrodes. In this case, the hydrogen electrode contains a metal hydride alloy, an oxide-nickel electrode is located between the hydrogen and oxygen (air) electrodes, and an alkaline electrolyte is taken as the electrolyte. The design of VKTEA described above allows to combine and integrate TE and nickel-metal hydride batteries in one housing. In this case, in view of the high reversibility of the hydrogen electrode, it is common for the FC and the battery. The combination of a battery and a fuel cell makes it possible to significantly increase the specific characteristics and resource of VKTEA.
It is expedient that the oxide-nickel electrode be separated from the hydrogen and oxygen (air) electrodes by separators. The presence of separators prevents the possibility of closing these electrodes. Advantageously, the oxide-nickel electrode is configured to be electrically connected to the oxygen (air) electrode, for example via a switch. The possibility of such a connection through this switch allows you to disconnect the oxygen (air) electrode when charging the battery and connect it parallel to the oxide-nickel electrode while simultaneously discharging the FC and battery.
It is expedient that the oxide-nickel electrode has a porosity in the range of 60-80%, and its relative resistance over the electrolyte is 1.5-8. The high porosity and low relative resistance over the electrolyte of the oxide nickel electrode located between the hydrogen and oxygen (air) electrodes VKTEA, will not lead to a significant increase in the internal resistance of VKTEA.
It is advisable that the metal hydride alloy is located on the surface of the hydrogen electrode facing the hydrogen chamber, and the charge capacity of the oxide-nickel electrode is matched with the charging capacity for the hydrogen of the metal-hydride alloy. Placing the alloy on the surface of the electrode facing the hydrogen chamber facilitates the sorption of hydrogen released during battery charging and desorption of hydrogen when the battery is discharged. The coordination of these capacities ensures optimal design of the battery and VKTEA as a whole.
The conducted analysis of the prior art showed that the claimed set of essential features set forth in the claims is not known. This allows us to conclude that it corresponds to the criterion of "novelty."
To check the conformity of the claimed invention, the criterion "inventive step" was carried out an additional search of known technical solutions in order to identify features that coincide with the features of the claimed technical solution that are distinct from the prototype. It was found that the claimed technical solution does not explicitly follow from the prior art. Therefore, the claimed invention corresponds to the criterion of "inventive step".
The essence of the invention is explained by the drawing and description of the structure of the claimed VKTEA
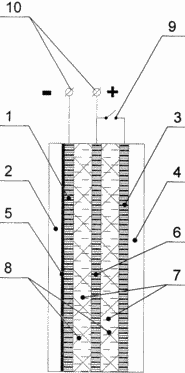 |
In the drawing, VKTEA is represented in the section. VKTEA includes a hydrogen gas diffusion electrode 1 with a hydrogen chamber 2, an oxygen (air) gas diffusion electrode 3 with an oxygen (air) chamber 4. A hydrogen electrode 1 on the surface facing the hydrogen chamber contains a metal hydride alloy 5. The oxide-nickel electrode 6 is separated from the hydrogen 1 and oxygen (air) 3 electrodes by separators 8. Hydrogen electrode 1 and oxygen (air) electrode 3 are separated by alkaline electrolyte 7. Oxygen (air) electrode 3 through switch 9 is connected to oxide-nickel electrode 6. When charging (discharging) VKTEA external The electric power source (load) is connected to the electrical terminals 10. When charging, the switch 9 is open and the battery is charged with electrodes 1 and 6. When discharged, the switch 9 is closed and the battery discharges with electrodes 1, 6 and the operation of the TE with the electrodes 1, 3. DETAILED DESCRIPTION OF THE INVENTION
|
CLAIM
1. A hydrogen-oxygen (air) fuel cell-battery (VKTEA) containing a hydrogen gas diffusion electrode with a hydrogen chamber, an oxygen (air) gas diffusion electrode with an oxygen (air) chamber and an electrolyte located between the hydrogen and oxygen (air) electrodes, differing In that the hydrogen electrode contains a metal hydride alloy, an oxide-nickel electrode is located between the hydrogen and oxygen (air) electrodes, and the alkaline electrolyte is taken as the electrolyte.
2. VKTEA according to claim 1, characterized in that the oxide-nickel electrode is separated from the hydrogen and oxygen (air) electrodes by separators.
3. VKTEA according to claim 1, characterized in that the oxide-nickel electrode is configured to be electrically connected to an oxygen (air) electrode.
4. VKTEA according to claim 3, characterized in that the oxide-nickel electrode is electrically connected to the oxygen (air) electrode by means of a switch.
5. VKTEA according to claim 1, characterized in that the oxide-nickel electrode has a porosity in the range of 60-80%.
6. VKTEA according to claim 1, characterized in that the relative resistance of the oxide-nickel electrode over the electrolyte is from 1.5 to 8.
7. VKTEA according to claim 1, characterized in that the metal hydride alloy is located on the surface of the hydrogen electrode facing the hydrogen chamber.
8. VKTEA according to claim 1, characterized in that the charging capacity of the oxide-nickel electrode is matched with the charge capacity of the hydrogen of the metal-hydride alloy.
print version
Date of publication 13.01.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.