| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2182737
![]()
An electrochemical fuel cell (optional), a membrane - electrode (variants) COMPOSITION (VARIANTS), METHOD FOR PRODUCING ELECTROCHEMICAL FUEL CELL AND METHOD FOR PRODUCING UNIT MEMBRANE - ELECTRODE (optional)
Name of the inventor: Robert D. Mussel (US); BABINEK Syuzn J. (US).; SKORTICHINI L. Carey (US); PLOUMAN Keith R. (US); Stephen P. Webb (US); Timothy J. REG. (US)
The name of the patentee: The Dow Chemical Company (US)
Address for correspondence: 129010, Moscow, ul. Boris Spassky, 25, p.3, Ltd. "Gorodissky and Partners", E.I.Emelyanovu
Starting date of the patent: 1996.10.04
The invention relates to electrochemical fuel cells and membrane assemblies - the fuel cell electrode. Said invention is to increase the specific electrical characteristics of the fuel cell. According to the invention, an electrochemical fuel cell has a membrane - electrode and adjacent to a layer of electrically conductive porous material having a porosity of at least 50% and an average pore size of at least 35 microns. The fuel cell system comprises at least three successive elements. A membrane - electrode has an ion exchange membrane and at least two active layers. A process for preparing an electrochemical fuel cell comprises applying the conductive composition layer on the porous conductive sheet to form a composite material and placing the composite adjacent to the membrane - electrode.
DESCRIPTION OF THE INVENTION
The invention relates to electrochemical fuel cells and more particularly to the membrane sites - the fuel cell electrode and allied structures flow field.
Electrochemical fuel cells generate electricity through fuel oxidation. One type of fuel cell uses the membrane unit - an electrode (MEA) comprising a membrane having an anode side and a cathode side depending on the direction of the current in relation to it. The membrane itself serves as an electrolyte. The corresponding electrochemical reaction catalyst is applied to the membrane, or introduced into the polymer composition from which the membrane is obtained. Alternatively, the catalyst is applied to carbon fiber paper which is then laminated to the membrane to form a membrane assembly - electrode.
On both sides of the MEA is a flow field which typically consists of a graphite plate which is processed to yield a series of channels on its surface, as shown for example in US Patents 5300370 and 5230966. As fuel channels fed to the anode side and oxidant to the cathode side and The reaction products are discharged mainly from the cathode side, and are typically separated from the membrane assembly - a thin electrode layer of porous carbon material such as carbon fiber paper.
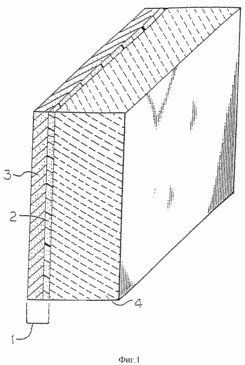 |
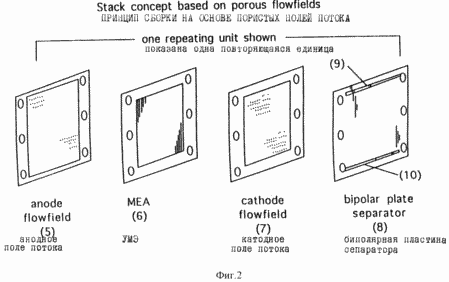 |
Figure 1 shows one embodiment node membrane - electrode and the flow field of the fuel cell of the first aspect of the invention. Figure 2 shows the structure of the repeating unit, which can be used to obtain a fuel cell stack comprising a plurality of fuel cells arranged in series, which imposes a membrane - electrode and a flow field shown in Figure 1.
 |
 |
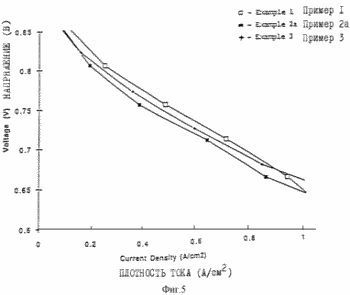 | |
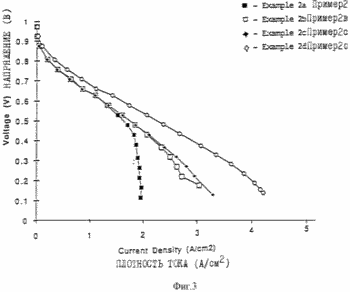
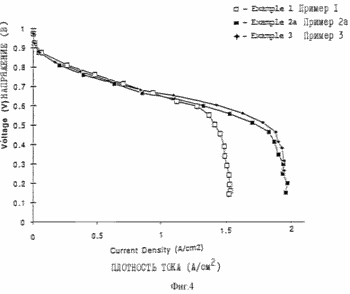
Figures 3, 4 and 5 illustrate the characteristics of the fuel cell with reference to Examples 1, 2 and 3.
 |
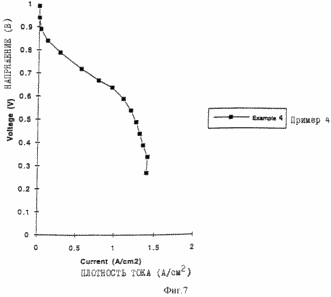 |
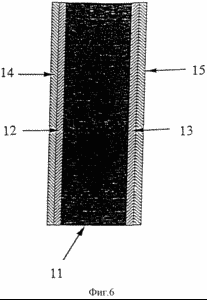
Figure 6 illustrates a membrane - electrode having two active layers positioned on the same side of the membrane.
Figure 7 shows the characteristics of membrane-electrode assemblies prepared as described in Example 4.
 |
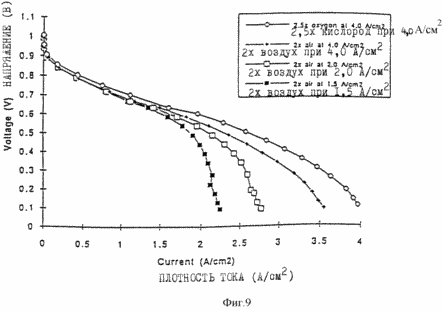 |
Figure 8 shows a membrane - electrode having a porous layer adjacent to it, and the flow field.
 |
 |
 |
 |
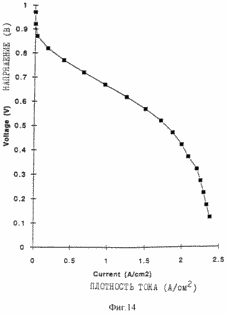 |
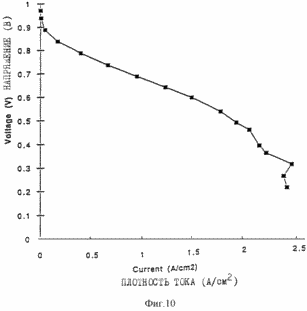
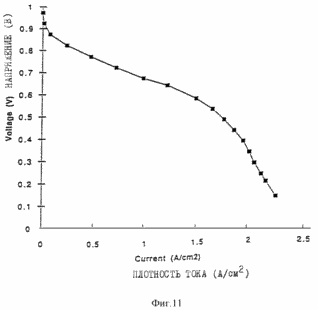
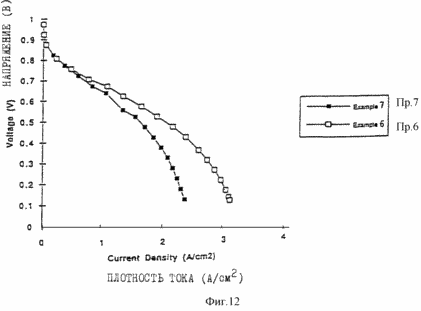
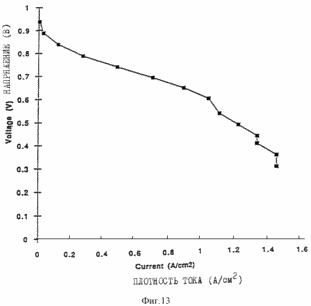
Figures 9, 10, 11 and 12 are of the fuel cells, which introduce the porous layers prepared as described in the examples. Figure 13 shows the characteristics of membrane-electrode assemblies described in Example 9. Figure 14 shows the characteristics of the membrane assemblies - electrode described in Example 10. In one aspect, the invention provides an electrochemical fuel cell having a membrane - electrode adjacent to it and the flow field, wherein the flow field comprises an electrically conductive material having a porosity of at least 50% and an average pore size of not less than <35 microns. It has been found that the fuel cell of the first aspect of the invention is able to operate at relatively high current densities and relatively high voltages at lower gas flow rates. |
Typically, in a fuel cell membrane and a polymer layer containing metal catalytically-active particles ( "active layer") must be hydrated in order to be sufficiently ionically conductive. During operation of the fuel cell on the cathode side of the active layer formed by the water that condenses in the adjacent flow field. Water may be present as a result of wetting of one or both of the reactant gases. However, if too much water condenses or accumulates adjacent to the active layer or the active layer, the coefficient of efficiency of the fuel cell is reduced, since diffusion of gas through liquid water is slow relative to its diffusion through water vapor.
It is believed that the characteristics of porosity and pore size of the flow field in the fuel cell of the first aspect of the invention to improve the mass transfer characteristics, which results in higher voltages at high current densities. It is believed, without intending to be bound by any particular theory, that the relatively high porosity and large pores effective in combination retain the gas transfer in the presence of water in the liquid state. Because the feed gas flow is in the plane of the flow field and substantially parallel to the active layer, the liquid is deflected from the active layer and the flow field is removed from the gas stream, thus keeping pores open for effective transport of reactant gas to the catalytically active particles. However, when the flow field is relatively thick (e.g., 0.5 mm for supplying air stoichiometry equal to 2 at 206.8 kPa), the gas velocity becomes inadequate to maintain clean pores of water. In such cases it is believed that increasing the wettability promotes flow creating such conditions that may be applied to an annular flow regime in which the liquid spreads onto the solid surfaces of the porous structure, leaving the centers of the large pores open and available for effective gas transport.
In a second aspect the present invention provides a membrane - electrode assembly having a solid polymer electrolyte, and at least two active layers positioned on the same side of the membrane, wherein the active layers comprise catalytically-active particles and an ionomer, wherein the average equivalent weight ionomers in the different layers not less than 50 and the active layer positioned closest to the membrane (hereinafter "first" active layer) contains the ionomer with the lower average equivalent weight. "Second" active layer (the layer positioned on the side of the first active layer which is reverse to the side facing the membrane) may be located either adjacent to and in contact with the first active layer, or one or more additional active layers may be positioned between the first and second active layers.
In a third aspect the present invention provides a membrane - electrode assembly having a solid polymer electrolyte, and at least one active layer positioned on one side of the membrane, wherein the active layer comprises (a) catalytically active particles, and (c) an ionomer having an equivalent weight in the range from 650 to 950 and which is substantially insoluble in water at temperatures less than 100 o C.
It was found that membrane components - an electrode (MEA), second and third aspects of the invention when used in a fuel cell, provide a relatively high voltage at a given current density and gas flow rate.
It is believed that the ionomer equivalent weight affects the water content of the active layer. It is believed, without intending to be bound by any particular theory, that the ionomer equivalent weight lower supports higher water content at low current densities. This higher water content improves the proton conductivity and the accessibility of the catalytically active particles, thereby increasing the voltage. However, this increase in water content may reduce the characteristics (voltage) at higher current planes. It has been found that the performance at both high and low current densities may be optimized by using a multi-layer active layer with the ionomer equivalent weight varying in each layer. It is believed, without intending to be bound by any theory, that the improved performance is the result of differences in hydrophilicity between the layers. It is believed that the ionomer with the lower equivalent weight, attached to the membrane, provides a zone in the MEA having a high water content, the best performance at low current densities, while the less hydrophilic ionomer of higher equivalent weight helps transport water away from the membrane at higher current densities . In a third aspect, the use of an ionomer having a relatively low equivalent weight gives better performance at lower current densities.
In a fourth aspect the present invention provides an electrochemical fuel cell having a membrane - electrode and a layer of electrically conductive porous material adjacent thereto which has but at least two portions with different mean pore sizes, wherein a first portion of the layer adjacent to the membrane - electrode has a porosity of not greater than the porosity of the second portion of the layer adjacent to the opposite side of the layer, the second portion has a porosity of at least 82%; and the second portion has an average pore size that is at least 10 microns and is not less than 10 times the average pore size of the first part.
In a fifth aspect the present invention provides an electrochemical fuel cell having a membrane - electrode with a nonwoven porous layer of an electrically conductive porous material adjacent thereto which has at least two portions with different mean pore sizes, wherein a first portion of the layer adjacent to the membrane ± node electrode has a porosity of not greater than the second portion of the layer adjacent to the opposite side of the layer; the second portion has a porosity of at least 50%; and the second portion has an average pore size that is not less than 35 microns and is not less than 10 times the average pore size of the first part.
In a sixth aspect the present invention provides a process for preparing an electrochemical fuel cell having a membrane - electrode, comprising the steps of:
(A) applying a layer of a conductive composition to a sheet of porous conductive material having a porosity of at least 82%, under conditions sufficient to form a porous solid layer conductive composition on one side of the sheet of porous conductive material, thereby forming a composite, and
(C) placing the composite adjacent to the membrane - electrode, so that the side of the composite to which the conductive composition was applied is facing said assembly.
It has been found that the fuel cells of the fourth and fifth aspects of the invention, as well as fuel cells prepared by the process of the sixth aspect of the invention are capable of operating at high current density at relatively high voltage, have a relatively high power density and provide a high power density even when operating at relatively low gas pressures.
In a seventh aspect the present invention provides a composition comprising (a) catalytically active particles, (c) an organic compound having a pK of at least 18 and basicity parameter ![]() less than 0.66, and (c) a polymeric binder.
less than 0.66, and (c) a polymeric binder.
In an eighth aspect the present invention provides a method for producing the membrane assembly - electrode which comprises the sequential steps of (i) applying a layer of the composition of the first aspect of the invention a solid polymer electrolyte, carbon fiber paper, or a release substrate; (Ii) compositions heating under conditions sufficient to volatilize at least 95% of component (c), and (iii) placing the composition in contact with a solid polymer electrolyte, if the composition was not applied directly to the solid polymer electrolyte, thereby forming a node membrane - electrode.
It has been found that the composition and method of the seventh and eighth aspects of the invention, when used for the membrane assembly - an electrode (MEA) having a solid polymer electrolyte, provides an MEA which provides a relatively high voltage at a given current density and gas flow rate in the fuel cell. It is believed, without intending to be bound by any particular theory, that the improved performance is the result of the ability of the organic compounds are easily volatilized when heated, which is believed to be the result of a small scope of ionic, hydrogen, or covalent bonds or partial bonds formed between the organic compound and a polymeric binder, especially when the binder is in an ionic form. Although the tendency of the organic compound to form a bond with the binder is difficult to quantify the stated above generalized characteristics of the organic compound is a defining characteristic, which is believed to have a connection to indicate little or no ability to form a bond with the ionomer or polar polymer. Indicator pK parameter mainly reflects acidic and basic compounds respectively.
It is believed that the ease with which the organic compound may be removed from the paste, greatly affect the porosity characteristics of the resulting active layer.
It is assumed that the easy removal of the organic compound contributes to "blowing" effect in the layer, which increases the porosity of the pore layer. Porosity characteristics affect the transport of water through the layer, which significantly affects the performance of the MEA into which it is introduced. Moreover, if the composition of the seventh aspect of the invention (hereinafter "catalyst paste") is applied directly to the membrane, it will not cause excessive swelling of her, since the organic compound will not be strongly associated with the ionomer membrane. Further, the composition of the invention allows the use of Na + - and H + - form ionomers as the binder without significant degradation it when the catalyst paste is heated to volatilization of the organic compound, and provides an active layer with good long-term stability.
These and other advantages of these inventions will be apparent from the following description.
Turning now to Figure 1 and to Figure 8, the term "unit membrane - electrode" (1), as used herein, refers to a solid polymer electrolyte combination (and called "membrane" here) and a catalytically active particles in the assembly of the fuel cell, regardless of its configuration or method of preparation. The layer of membrane material containing such particles are referred to as "active layer", regardless of whether such particles are introduced into the discrete layer of polymer (2) and applied or laminated to whether the surface of the membrane (3), or introduced into the membrane itself. Turning now to Figure 1, the flow field (4) is a porous layer of an electrically conductive material and having an input connected to the output of the gas flow. flow field may comprise porous carbon material. The fuel cell of the first aspect of the invention preferably comprises a flow field leaking components having slotted, crushed or molded flow channels extending along their entire active surface. These channels deliver gases directly to the active layer through a porous carbon "substrate layer" which supports the active layer, as shown in Figures 1 and 4 of US Patent 5108849. However, the flow field, bipolar plates and / or end plates used as supports for fuel element and the separation of elements in a multiple structure may contain one or more channels to increase the flow of reactant gases to the flow field in the fuel cell of the first aspect of the invention. An example of these channels is shown in Figure 2.
Examples of suitable porous carbon materials which may be used as the flow field in the first aspect of the invention include carbon paper, graphite paper, carbon felts, or other carbon-containing composites which contain at least 20 wt.% Carbon. flow field can be intertwined channels cut into it to lower the pressure drop introduced into the reactant gases. If desired, the porous carbon material may be treated or perftorsilanom fluorochemical composition to increase its hydrophobicity, or oxidized, sulfonated, or coated with a hydrophilic material to increase its hydrophilicity. If the flow field has a thickness of not less than 0.5 mm, it preferably has a relatively high wettability. The wettability of the flow field may be determined experimentally as follows: a sample of the flow field size 25.4 x 25.4 mm vertically kept in a tray containing water, a depth of 4.76 mm. The amount of water soaked for 10 seconds, determined by weighing. Flow field more preferably 0.5 mm to absorb at least 0.5 g of water per 1 g of the porous material, more preferably not less than 1 g of water per 1 g of the porous material.
The conductivity of the flow field layer in the first aspect of the invention is preferably at least 0.01 S / cm, more preferably at least 0.1 S / cm and most preferably at least 0.1 S / cm. The preferred thickness of the flow field depends on the optimal pressure limit across the flow field, but is preferably not less than 0.025 mm, more preferably not less than 0.127 mm and most preferably not less than 0.254 mm, but preferably not more than 6.35 mm, more preferably not more than 2 54 mm, and most preferably not more than 1.27 mm. The porosity of the flow field is preferably at least 75%, more preferably at least 80%. The average pore size of the flow field is not less than 45 microns, more preferably not less than 50 microns, but is preferably not more than 250 microns. The term "average pore size" used herein means that half the free volume of the material contained in pores larger in diameter than the average pore size, and half is contained in pores equal to or smaller than the average pore size. The average pore size can be determined by any convenient method such as mercury porosimetry. The device used to determine the distribution of mid-sized bed then, can be calibrated using a calibration standards based on silica / alumina (supplied by Mikrometriks, Norcross, GA).
All VME described herein may be prepared in any suitable manner, unless otherwise specified. In one method, the catalytic layer "paste" is first applied to a solid polymer electrolyte, carbon fiber paper, or release substrate. Catalyst paste typically comprise catalytically-active particles (such as platinum deposited on carbon), binder, solvent or dispersing agent that provides a uniform application of a thin layer of the catalyst-ionomer mixture to the solid polymer electrolyte, carbon fiber paper, or release substrate.
For the second aspect VME catalytic layer "paste" is first applied to a solid polymer electrolyte, and then a second and third layer of paste is applied to the part of the MEA located opposite the first active layer or to a release substrate, or on top of the first active layer. As used herein, the term "active layer" refers to a layer comprising a mixture of ionomer and catalytically-active particles.
MEA in the fuel cell of the first aspect of the invention may be prepared by any suitable method, but preferably is obtained by applying the catalyst ink (a suspension or dispersion of the catalytically active particles) directly to a solid polymer electrolyte as described, for example, in US Patent 5211984. The paste is applied to the membrane for one or more drawing operations sufficient to obtain the desired content of the catalytically active particles. Preferably the catalytically active layer is obtained by applying particles of at least two separate stages of pastes to form the various layers on the paste with a paste binder with the highest equivalent weight applied so that it is located adjacent to the flow field in the fuel cell. In such cases, a membrane - electrode comprises a solid polymer membrane having at least two layers of catalyst ink on at least one side thereof, wherein at least two catalyst ink layer containing polytetrafluoroethylene polymers having pendant sulfonic acid groups , equivalent weights which differ by more than 50, and wherein the layer having the highest equivalent weight is positioned adjacent to the flow field. Immediately after receiving the MEA is located near the flow field in the fuel cell assembly.
Fuel cells described herein may be administered in a multiple structure or "pack" containing a number of fuel elements, preferably at least three successively arranged. Example repeating unit represented in figure 2, which shows an anode flow field (5), MEA (6), a cathode flow field (7), and a bipolar separator plate (8). The bipolar separator plate has ducts (9) and (10) that transport the reactants and reaction products to and from the flow field. In this design, MEA is disposed between two porous flow fields that have an inert material impregnated into peripheral regions (the darker bands in the figure) to prevent the exit of reactant gases to the outside. The holes in peripheral portions of all the elements together form a gas manifold when they are collected together and placed under pressure. The material used for the bipolar plate separator may be selected from a number of rigid and non-rigid materials, and the plate has gas delivery channels molded or cut into its surface. These channels are fed reactant gases to the porous flow fields and withdrawn from the reaction products of the past. In an alternative embodiment, gases and products may be introduced or output via channels or void spaces in the porous flow field connected to manifolds. The bipolar separator plate, and can have an internal structure for circulating a cooling fluid therein.
If the paste, which is obtained from the second active layer is deposited on top of the first active layer, the first active layer is preferably first dried sufficiently before application of the second paste to prevent too much mixing of pastes. However, a small degree of mixing of pastes in their point of contact with each other may be desirable since it promotes electrical and ionic conductivity between the layers. After applying the paste are preferably heated under conditions sufficient to volatilize at least 95% of any amounts of organic solvent or dispersant present in the pastes.
As used herein, the term "solid polymer electrolyte" refers to a porous layer, consisting of a solid polymer which has a porosity of not less than 1 × 10 -3 S / cm under operating conditions of the fuel cell or electrolytic cell, or which may be reacted with an acid or a base to form a porous layer having such conductivity. Preferably, the solid polymer electrolyte comprises a film of a sulfonated fluoropolymer, or a layered composite of films of sulfonated fluoropolymers having different equivalent weights.
After applying the catalyst ink to a solid polymer electrolyte paste is preferably heated under conditions sufficient to remove a sufficient amount of an organic solvent or dispersant, so that the active layer comprises at least 99 wt.%, More preferably at least 99.9 wt.% Of a mixture of catalytically active particles and ionomer. The paste is applied in an amount sufficient to produce a mixture layer which has a thickness of not less than 1 m, more preferably not less than 5 microns, and most preferably at least 10 microns; but is preferably no more than 30 microns. The porosity of the layer is preferably at least 30%, more preferably at least 50%, but preferably not more than 90%, more preferably not more than 60%. The average pore size of the layer is preferably at least 0.01 .mu.m, more preferably not less than 0.03 microns; but is preferably no more than 10 microns, more preferably less than 0.5 microns, and most preferably 0.1 microns. The above characteristics of thickness, porosity and pore size measurements are performed when the ionomer (ionomers) contained in the layer are in their dry and protonated form.
Then components VME second and third aspects of the invention are collected by placing one of the active layers in contact with a solid polymer electrolyte, and then positioning the second and third active layer so that it is between the first active layer and the porous carbon material, thereby forming a node membrane - electrode .
As used herein, the term "catalytically active particles" refers to particles of a metal or compound which is catalytic for the electroreduction of oxygen or electrooxidation of hydrogen or methanol under the influence of pressure and temperature in the fuel cell. Examples of such particles used comprise particles of platinum, ruthenium, gold, palladium, rhodium, iridium, their electroconductive and reduced oxides and alloys of such materials, either in combination with each other or with other transition metals, particles may be coated onto the respective material, if required, such as carbon black. Preferably, the catalytically active particles are platinum particles supported on carbon, which preferably contains from 10 to 30 wt.% Of platinum. The size of the catalytically active particles (without substrate) is preferably at least 10 ![]() , More preferably at least 20
, More preferably at least 20 ![]() But preferably not more than 500
But preferably not more than 500 ![]() , More preferably not more than 200
, More preferably not more than 200 ![]() . It can be used and larger particles, or may be formed during work with small particle agglomeration. However, the use of such particles may result in decreased cell performance.
. It can be used and larger particles, or may be formed during work with small particle agglomeration. However, the use of such particles may result in decreased cell performance.
The catalytically active particles are preferably used in an amount sufficient to provide an optimum catalytic effect in the operating conditions of the electrochemical device in which they are applied. Preferably, they are used in an amount sufficient to provide the level of the cathode side of the membrane at not less than 0.05 mg / cm 2, more preferably not less than 0.1 mg / cm 2, and most preferably not less than 0.15 mg / cm 2; but preferably not more than 0.45 mg / cm 2, more preferably not more than 0.35 mg / cm 2, and most preferably not more than 0.25 mg / cm 2. The content on the anode side of the membrane is preferably at least 0.01 mg / cm 2 but not more than 0.15 mg / cm 2. Relative to the amount of ionomer, however, the particles are preferably present in the paste in an amount by weight of the catalytic particles, including their substrate (if there is any) sufficient to provide a weight ratio of particles: ionomer of at least 2: 1, but preferably not more than 5: 1.
Examples of suitable organic compounds for use in the preparation of the catalyst ink (except for the paste of the seventh aspect of the invention) include polar solvents such as glycerin, C 1-6 alcohols, and other compounds such as ethylene carbonate, propylene carbonate, butylene carbonate, etilenkarbamat, propilenkarbamat, butilenkarbamat acetone, acetonitrile, difluorobenzene, and sulfolin, but most preferred is propylene. The organic compound is preferably present in an amount relative to the weight of the composition of at least 10%, more preferably at least 20% and most preferably at least 30%, but preferably not more than 90%. Such solvents in the ink function primarily as solvents or dispersants.
Suitable ionomers for use in the preparation of catalyst pastes described herein include any polymer or oligomer having ionic conductivity of at least 10 -3 S / cm, more preferably not less than 10 -1 S / cm (under the operating conditions of the fuel cell or electrolyser) or which can be reacted with an acid or a base to form an oligomer or polymer having ionic conductivity.
Examples of suitable ionomers include fluoropolymers having pendant ion exchange groups such as sulfonic acid groups in proton or salt form. Examples of such polymers included fluorosulfonic having fluoropolymer backbones and side ion exchange groups with carbon number of 1 to 5 attached thereto and terminating in sulfonyl group are suitable for use in the present invention. Examples of such fluoropolymers with sulfonic ion exchange are shown, for example, in US patents 4578512, 4554112, 4515989, 4478695, 4470889, 4462877, 4417969, 4358545, 4358412, 4337211, 4337137 and 4330654.
Preferably, the ionomer has a substantially fluorinated polymer backbone and recurring pendant group having the general formula
-O- (CFR) a - (CFR ') -SO sin M,
wherein a and b are independently 0 or an integer from 1 to 3, and (a + c) - at least 1;
R and R 'are independently selected from halogen, perfluoroalkyl and fluorochloroalkyl; and M is independently selected from hydrogen or an alkali metal.
Other ionomers used in forming both thick and thin composite membrane layers are characterized by a substantially fluorinated polymer backbone and recurring pendant group having the general formula
O- (CFR) a - (CFR ') a -O- (CF 2)c -SO 3 M, (II)
wherein a and b are independently 0 or an integer from 1 to 3, with - an integer from 1 to 3; (A + b) is at least 1; R and R 'are independently selected from perfluoroalkyl, halogen, and fluorochloroalkyl and M are independently selected from hydrogen or an alkali metal.
Ionomers having the above formula are discussed in US Patents 4478695, 4417969, 4358545, 4940525, 3282875 and 4329435. The ionomer is preferably present in an amount relative to the weight of the composition of at least 0.5% but preferably not more than 5%. The ionomer may be used in any ionic form, such as the proton form or salt form of the corresponding oligomer or polymer. Examples of salt forms include quaternary ammonium, sodium, lithium and potassium.
In a second aspect of the invention ionomers are used to obtain pastes preferably have an equivalent weight based on the number of side ionic groups per molecule, at least 600, more preferably not less than 700 and preferably not more than 1,200, more preferably not more than 950., However, ionomer and must be substantially insoluble in water at temperatures below 100 o C, so the equivalent weight of some fluoropolymers can be higher. As used herein, the term "substantially insoluble in water" means that the pure ionomer in the ionic form is at least 75% insoluble in distilled water at any concentration. The difference between the equivalent weight of the ionomers in at least two pastes used to prepare the MEA is preferably at least 50, more preferably at least 100, and most preferably at least 300, but preferably not more than 800, more preferably not more than 600, and most preferably not more than 400. In the second and third aspects of the invention, the ionomer used to prepare the paste preferably has an equivalent weight of at least 650, more preferably at least 700 and most preferably at least 770, but preferably not more than 950, more preferably not more than 900 and most preferably not more than 840. The equivalent weight of the ionomer can be determined by any suitable method, such as shown in US Patent No. 4,940,525.
Regarding now the figure 6, which shows a membrane - electrode of the second aspect of the invention, shows the membrane (II), having two active layers positioned on each side of the membrane. The active layers closest to the membrane (12, 13) contain ionomers having lower average equivalent weights than the active layers positioned adjacent thereto (14, 15).
Regarding now the figure 8, which shows a membrane - electrode of the fourth and fifth aspects of the invention, the porous layer (16) is a layer of electrically conductive porous material having at least two portions with different mean pore sizes, which is disposed between the active layer and flow field. flow field (17) may comprise a mechanically machined graphite plate, or may mainly consist of a thick layer of porous carbon material as described, for example, in US Patent 5252410. However, the porous layer (16) does not contain any catalysts which are normally present in the active layer, such as platinum.
The fuel cells of the fourth and fifth aspects of the invention comprise a porous layer of conductive material (hereinafter the "intermediate layer"), which is adjacent to the membrane - electrode and has at least two portions with different mean pore sizes. Portion of the layer adjacent to the membrane - electrode (18) (hereinafter "zone of small pores"), has an average pore size that is at least 10 times less than that of the layer adjacent to the opposite side of the layer (19 ) (hereinafter "the zone of large pores").
Compositions suitable for use in the preparation of the intermediate layer include any organic or inorganic composition which can be manufactured in a solid layer having the characteristics of porosity and pore size as described above and which and has sufficient dimensional, hydrolytic and oxidative stability under the operating conditions of the fuel element. One way to obtain an intermediate layer having asymmetric pore size characteristics is to obtain such a layer from two or more materials having different mean pore sizes. An example of such a method is to first obtain or prepare a material having an average pore size appropriate for the zone of large pores (hereinafter "the material with large pore"), and then the impregnation and / or coating one side of the material composition that reduces the porosity of the material is sufficient to obtain the smallest desired pore size and / or forming a discrete layer of the composition on the outer side of the material having the desired small pore characteristics.
Typically, in a fuel cell membrane and a polymer layer containing a metal catalyst ( "active layer") must be hydrated in order to be sufficiently ionically conductive. During operation of the fuel cell on the cathode side of the active layer formed by the water that condenses in the adjacent flow field. The water may be present due to the humidification of one or both of the reactant gases. However, if too much water condenses or otherwise accumulates adjacent to the active layer or the active layer, the efficiency of the fuel cell is reduced, since diffusion of gas through liquid water is slow relative to its diffusion through water vapor.
It is believed, without intending to be bound, that the area of fine pores reduces the accumulation of excess liquid water in the active layer because it serves as a semi-permeable layer or membrane which allows water vapor formed in the active layer or present in the result of humidification of the reactant gases to pass between the active layer and the flow field, but reduces or prevents condensation of water on the active layer and prevents the liquid water present in the flow field or large pore area of the intermediate layer back through the passageway from the zone of small pores to the active layer. Preferably, the wettability (determined by the pore size and the angle of wetting water - solid) zone of small pores is such that for a sufficiently large fraction of long discharge pressure required for injecting liquid water into these pores is larger than the hydraulic pressure in the flow field components under the prevailing pressure and temperature conditions in the fuel cell.
Examples of suitable organic compositions which may be used to obtain or impregnating a large pore material include thermoplastic or thermosetting polymeric or oligomeric materials, such as polytetrafluoroethylenes, including those which have sulfonic acid groups (such as Nafion, supplied by Du Pont), polyalkylene oxides, polyolefins, polycarbonates benzotsiklobutany, perfluorocyclobutane, polyvinyl alcohols, and polystyrene, epoxy resins, and perftoralkilakrilovye copolymers, polyanilines, polypyrroles, and mixtures thereof. Preferably the composition is a polytetrafluoroethylene, a copolymer or perftoralkilakrilovy perfluorocyclobutane and most preferably perfluorocyclobutane. Examples of suitable inorganic compositions which may be used include clays, silicates, and titanium-containing composition.
The compositions used for small pore zone intermediate layer, preferably comprise a polymer, carbon particles and a suitable carrier. The carrier normally impregnates the entire large pore material, although the bulk of the polymer and the carbon particles collected at or near the surface of the fabric to which it is applied (depending on the porosity and size of particles contained in the composition), thereby forming a finely porous area on the side of the material on which the composition is applied. Consequently, the area or portion of the intermediate layer having different mean pore sizes are not necessarily discrete layers, because at least the first 1 micron depth of the small pore region and at least the first 50 microns depth macroporous zone (measured from the bed surface direction perpendicular to the layer) has the necessary pore characteristics.
The intermediate layer may be obtained by applying the composition used in the preparation of the small pore region on; a membrane - electrode, and then the placement or lamination layer of large pore material adjacent to it. Alternatively, the film of the composition used in the preparation of the small pore region may be prepared separately using conventional technologies for films and then positioned or laminated between the membrane assembly - electrode and a large pore material. If the composition is applied to the MEA, it may be applied using any suitable coating method such as painting or screen printing.
Zone microporous intermediate layer is preferably at least as hydrophobic as the active layer. The composition used to prepare the small pore region is preferably zhidkostsoderzhaschey composition which solidifies after application. If the composition which is used is the solvent containing a sufficient amount of the solvent is removed to form a solid layer of material to the fuel cell assembly. Such solvent may be removed either at ambient conditions or at elevated temperatures. If appropriate, the composition is heated to increase its stability and uniformity, such as with crosslinked, or molecular weight increase agglomeration of the latex particles.
If the composition used to prepare the small pore region, should be applied directly onto a membrane - electrode, the bulk of the dissolved solids contained therein (such as polymer) is preferably hydrophilic in nature, since the membrane and active layer are usually obtained from a hydrophilic composition generally it is expected that applying the solution mainly hydrophobic dissolved solids properties of the active layer deteriorates. However, the composition used to prepare the small pore region is still preferably hydrophobic after being cured.
Hydrophobic fillers, such as carbon fibers and / or powders treated with hydrophobic compositions such as silan- and fluorine-containing composition can be used in compositions that are used to obtain small pore region to give it a hydrophobic nature and the deterioration of the wettability of its pores, and while increasing the porosity and average pore size of the solidified composition. In such cases, the weight ratio of carbon fibers or powders, and other components in the composition is preferably at least 1: 1, more preferably at least 3: 1, and preferably not more than 10: 1, more preferably not more than 5: 1 and most preferably 3: 1 . If the zone is obtained microporous coating composition macroporous material such as graphite paper is relatively fine pore structure of the paper will help keep the main part of the fillers in the composition close to the surface on the side of the paper on which it is applied. Alternatively, the composition may be a composition which is primarily hydrophilic as applied, but hydrophobic upon curing, such as a polytetrafluoroethylene latex. If the zone is obtained by applying microporous hydrophilic macroporous material composition, the strongly thin coating of material such as Zonil 7040 perftoralkilakrilovogo copolymer sold by DuPont, it may be applied to the side of the small pore region facing the MEA to further increase its hydrophobicity. Other examples of highly hydrophobic materials include Fluorad FC722 and FC724, supplied by ZM.
VME fourth and fifth aspects of the invention are preferably prepared by applying the catalyst ink (a suspension or dispersion of catalyst) directly to the membrane as described. For example, U.S. Patent No. 5211984. When the catalyst is applied on a porous carbon material, the composition used to prepare the small pore region is preferably applied first, followed by the catalyst paste, so that the impregnated porous carbon material may be used as an intermediate layer, and as a catalyst carrier layer. However, this method, and any methods which require a separate film for the intermediate layer are less preferred since such films and catalyst-containing structures must typically be laminated to the membrane portion of the membrane assembly - electrode to assemble a fuel cell. Such lamination processes, which employ heat and / or pressure applied to the excess intermediate layer, may alter or destroy its porous structure.
Furthermore, the composition of the intermediate layer may be formulated to optimize the maximum voltage at which the fuel cell will operate at a given current density. It is believed that higher voltages at higher current density require the microporous zone was more hydrophobic than at lower current densities. For example, to a higher voltage at a lower current density compositions having a higher ratio of carbon: polymer (such as 5: 1) are preferred for use in the preparation of the small pore region, particularly when applied to a graphite paper having a relatively low porosity . Similarly, if preferred are higher voltages at higher current densities are preferred lower ratio of carbon to polymer (such as 3: 1), especially when applied to a graphite paper having a relatively high porosity.
Microporous region preferably has a thickness in the range of 1 to 150 microns (as measured in a direction perpendicular to the intermediate layer) and has the desired characteristics of porosity and pore size. More preferably, the region has a thickness ranging from 5 to 25 microns. Preferably, the portion of the region adjacent to the MEA is sufficiently porous to allow the transmission of water vapor through the region. The porosity of this portion of the zone is preferably at least 10%. The average pore size of the small pore region is preferably at least 0.1 m, more preferably not less than 1 micron, but preferably less than 10 microns. The average pore size can be determined by any convenient method such as mercury porosimetry. The device used to determine the distribution of mid-sized bed then, can be calibrated using a calibration standards based on silica / alumina (supplied by Mikrometriks, Norcross, GA).
The term "average pore size" used herein means that half the free volume of the material contained in pores larger in diameter than the average pore size, and half is contained in pores equal to or smaller than the average pore size. The porosity of the small pore region is preferably at least 10%. To achieve the desired porous structure in the composition may be administered non-conductive and conductive fillers, inert fillers or fragile. As such conductive polymers such as doped polyaniline or polypyrrole, and can be used to prepare the composition in order to increase its conductivity. The porous structure of the small pore region may be adjusted to some degree using a choice of a polymer or oligomer composition.
Large pore region preferably has a thickness of not less than 0.051 mm, more preferably not less than 0.155 mm, but preferably not more than 1.27 mm. The porosity of this zone is preferably at least 82%, more preferably at least 85% and most preferably
not less than 87.5%. The average pore size of macroporous zone is preferably not less than 30 microns. The above values of porosity and pore size characteristics are the small pore region to a depth of at least 1 mm from the side of the intermediate layer approaches the MEA, and a depth of not less than 50 m from the opposite side of the intermediate layer, regardless of its method of preparation.
Examples of suitable porous carbon materials which may be used as the large pore material include carbon paper, graphite paper, carbon felts, or other carbon-containing composites which contain at least 20 wt.% Carbon. If desired, the porous carbon material may be treated or perftorsilanom fluorochemical composition to increase its hydrophobicity, or oxidized, sulfonated, or coated with a hydrophilic material to increase its hydrophilicity. If used porous carbon material, how the flow field and the large pore material may have interlocking channels cut in them to reduce the pressure drop introduced into the reactant gases. The conductivity of the intermediate layer is preferably at least 0.01 S / cm, more preferably at least 0.1 S / cm and most preferably at least 10 S / cm. The conductivity of the layer can be increased by the introduction of conductive fillers such as carbon fibers or particles or by introducing a conductive salts or polymers.
As used herein, the term "catalytically active particles" refers to particles of a metal or compound which is catalytic for the electroreduction of oxygen or electrooxidation of hydrogen or methanol under the pressure and temperature increase in the fuel cell. Examples of such particles used include particles of platinum, ruthenium, gold, palladium, rhodium, iridium, electroconductive and reduced oxides and its alloys such materials or in combination with each other or with other transition metals. The particles can be deposited onto a substrate of suitable material, if desired, such as carbon black. Preferably, the catalytically active particles are platinum particles supported on carbon, which preferably contains from 10 to 30 wt.% Of platinum. Size of catalyst particles (excluding the substrate) is preferably at least 10 ![]() , More preferably at least 20
, More preferably at least 20 ![]() But preferably not more than 500
But preferably not more than 500 ![]() , More preferably not more than 200
, More preferably not more than 200 ![]() . The particles are preferably used in an amount sufficient to provide an optimum catalytic effect under the operating conditions of the electrochemical device in which they are used. However, as the amount of binder, the particles in the paste are preferably present in an amount by weight of the catalytic particles, including their support, if any, sufficient to provide a weight ratio of component (a): component (c) at least 2:1, but is preferably no more than 5: 1.
. The particles are preferably used in an amount sufficient to provide an optimum catalytic effect under the operating conditions of the electrochemical device in which they are used. However, as the amount of binder, the particles in the paste are preferably present in an amount by weight of the catalytic particles, including their support, if any, sufficient to provide a weight ratio of component (a): component (c) at least 2:1, but is preferably no more than 5: 1.
Suitable organic compounds include organic compounds having a pKa (the negative logarithm of the equilibrium constant K of the reaction between the compound and water) of at least 18 and basicity parameter ![]() less than 0.66. Preferably the pK equal to 25. Preferably,
less than 0.66. Preferably the pK equal to 25. Preferably, ![]() It is less than 0.48, and more preferably less than 0.40. Basic parameters for a number of organic compounds, but also the existing method of determining it are described in Kamlet et al. "Linear Solvation Energy Relationships. 23 A Comprehensive Collection of the Solvochromatic Parameters, n *,
It is less than 0.48, and more preferably less than 0.40. Basic parameters for a number of organic compounds, but also the existing method of determining it are described in Kamlet et al. "Linear Solvation Energy Relationships. 23 A Comprehensive Collection of the Solvochromatic Parameters, n *, ![]() and
and ![]() and Some Methods for Simplifying the Generalized Solvatochromic Equation ", J. Org. Chem. Vol. 48, pp2877-2887 (1983).
and Some Methods for Simplifying the Generalized Solvatochromic Equation ", J. Org. Chem. Vol. 48, pp2877-2887 (1983).
Preferably, the compound volatilizes at temperatures in the range from 100 to 250 o C without significant degradation which can impair the characteristics of the active layer. Relatively low temperatures and volatilization is preferred, since organic compounds (component (c)) that are not removed from the layer, may increase the electrical resistance of the layer, causing poorer performance over VME. These characteristics are particularly important when the binder is used in its proton form, since the binder will act as a catalyst, facilitating further degradation of any residual organic compound. However, use of a proton form of the binder has advantages, since quaternary ammonium cations present in the paste composition are difficult to remove and may contribute to a long period of "running" when a fuel cell or fuel cell starts to work. Preferably, the boiling point of the solvent exceeds 100 o C, so that upon curing of a paste is first removed water and low boiling solvents which may be present in the ink (typically introduced in the form of a paste of a commercially available binder containing such components).
Examples of organic compounds suitable for use as component (b) include ethylene carbonate, propylene carbonate, etilenkarbamat, propilenkarbamat, butilenkarbamat, acetone, acetonitrile, difluorobenzene, and sulfolane, but is most preferably propylene carbonate. The organic compound is preferably present in an amount by weight of the composition of at least 10%, more preferably at least 20% and most preferably at least 30%, but preferably not more than 90%.
Suitable polymeric binders for use in preparing the compositions of the invention include any polymer or oligomer having ionic conductivity of at least 1 x 10 -3 S / cm, more preferably not less than 10 -1 S / cm (under the operating conditions of the fuel cell or electrolyser) or which may be reacted with an acid or a base to form an oligomer or polymer having ionic conductivity. If the binder has lateral ionic groups, it preferably has an equivalent weight of at least 600, more preferably not less than 700 and preferably not more than 1,200, more preferably not more than 950. The equivalent weight of the binder is one side of ionic groups per molecule, as can be determined by any suitable method such as titration with a base as shown in U.S. Patent No. 4940525. Examples of suitable binders include perfluorinated polymers and polytetrafluoroethylene polymers, and polytetrafluoroethylene polymers having pendant sulfonic acid groups (such as Nafion available from DuPont). Binder is usually present in an amount by weight of the composition of at least 0.5% but preferably not more than 5%. One advantage of the present invention is that the ionomer may be used in any ionic form, such as the proton form or salt form of the oligomer or polymer. Examples of salt forms include quaternary ammonium, sodium, lithium and potassium.
MEA may be prepared by any suitable method, including the method of the second aspect of the invention. Preferably the MEA is produced by applying one or more layers of the catalyst ink (the composition of the invention) directly to the solid polymer electrolyte as described, for example, in US Patent 5211984. As used herein, the term "solid polymer electrolyte" refers to a membrane consisting of a solid polymer which has conductivity of not less than 1 × 10 -1 S / cm under operating conditions of the fuel cell or electrolytic cell, or which may be reacted with acid or base to generate a membrane having such conductivity. Preferably, the solid polymer electrolyte comprises a film of a sulfonated fluoropolymer. Another method comprises applying one or more layers of the catalyst ink on a releasable material such as a polytetrafluoroethylene-coated substrate, curing the paste, and then laminating the cured material to the membrane. A third method comprises applying one or more layers of the catalyst ink to one side of a sheet of porous carbon material such as carbon or graphite paper, and then the location of the fabric to which the paste has been applied adjacent to the membrane. If the paste is cured before the location adjacent to the membrane, it should then preferably be laminated to the membrane to ensure good contact therebetween.
The paste can be cured by any suitable method for removing at least 95% of component (c) and and any other volatile organic solvents contained in the paste, such as by heating at an elevated temperature, optionally under reduced pressure. Preferably, the paste is heated at a temperature at which the component (a) evaporates, but below its boiling point. When producing the active layer MEA is used more than one paste preferably pastes contain as binder polytetrafluoroethylene polymer having pendant sulfonic acid groups, and a paste layer, closest to the membrane has an equivalent weight which differs from the equivalent weight of the binder in the layer paste adjacent to them not less than 50. In addition, a layer having a binder with the lowest equivalent weight preferably is located adjacent the solid polymer electrolyte.
Preferably, the paste is heated under conditions sufficient to remove at least 99%, more preferably at least 99.9% of component (c). The paste is applied in an amount sufficient to make the composition layer, which after drying has a thickness of protonation of less than 1 micron, more preferably not less than 5 microns, and most preferably at least 10 microns but preferably no more than 30 microns. The porosity of the layer is preferably at least 30%, more preferably at least 50%, but preferably not more than 90%, more preferably not more than 60%. The average pore size of the layer is preferably at least 0.01 .mu.m, more preferably not less than 0.03 microns, but preferably no more than 10 microns, more preferably less than 0.5 microns, and most preferably 0.1 microns.
The following examples are given to illustrate the invention and should not be interpreted as limiting it in any way. Unless otherwise indicated, all parts and percentages are by weight.
EXAMPLE 1
The membrane and electrode structures are obtained as follows:
Obtained ion-exchange membrane made of persulfokislotnogo ionomer having equivalent weight 800, 60 microns (2.4 mils) when dry and a thickness of 127 microns (5 mils) in the fully hydrated state (supplied by The Dow Chemical Company under the trademark 13204.20 HIS) , is cut into sheets 11 cm by 11 cm and placed into a NaOH bath to convert in Na + -form. The electrode paste was prepared by mixing 1.08 g of a 5.79 wt% solution of the above ionomer (in a solution of ethanol: water 50: 50% by volume)., 0.1875 g of 20% platinum on carbon (available from E-TEK, Natick, MN), and 0.114 g of a 1M solution of tetrabutylammonium hydroxide (TBAOH, plasticizer) in methanol, and 0.6 g of propylene carbonate (dispersing agent). The mixture was stirred stirred overnight or until until it is uniformly dispersed. To the mixture is added 1.2 g of propylene carbonate.
The catalyst paste is applied on clean card 9 cm2 fiberglass coated with polytetrafluoroethylene (brand SiEyAr Industries, New Haven, CT), which is dried in an oven at 110 o C and pre-weighed. Double-covered cards catalytic paste, which is completely dried before applying the second layer. The platinum content is 0.14 mg / cm2 on the anode and 0.25 mg / cm2 on the cathode. MEA is formed by coupling the coated blank on each side of the ionomer membrane that is dried on a vacuum table. Card and the membrane are placed between two stainless steel plates with holding them as they are placed in the press. The package is placed in a press at 195 o C and pressed at a pressure of 445 N per 1 cm 2 of the card for 5 min. Pressing the press is cooled to room temperature before opening. The card is peeled from the layer containing catalytically-active particles, leaving the film adhered to the surface of the membrane. Cathode flow field is carbon paper having a porosity of 90% and a thickness of 0.62 mm (as paper supplied by Spektrakarb Spectracorp, Lawrence, MA). The wettability of the paper is increased by oxidation in a medium comprising 0.006 M silver sulfate, 0.2 M sodium persulfate and 0.5 M sulfuric acid at 60 o C for 1 hour. The sample thus oxidized paper measuring 76.2 x 76 2 mm on standing it vertically in the cup to a depth of 4.76 mm, 2.7 g of water is absorbed per 1 g of carbon. Anode flow field is carbon paper having a porosity of 79% and a thickness of 0.356 mm.
A membrane - electrode and porous flow field tested in a test fuel cell obtained by Fuel Cell, Technologies, Inc.. (Santa Fe, New Mexico). MEA and flow field are placed between two solid graphite blocks, each with a single gas delivery channel and one outlet channel. The element is mounted on a singleton test stand made by Fuel Cell Technologies; Inc.. Flow field characteristics shown in Figures 4 and 5 in the flow conditions shown in the table. Unless otherwise indicated in the table, as the oxidizing gas air is used.
EXAMPLE 2
MEA / electrode structure was prepared using the procedure described in Example 1, except that the electrode paste was prepared by mixing 1 g of a 5% Nafion solution (polytetrafluoroethylene having sulfonic acid groups and an equivalent weight of 1100, available from DuPont), 0.130 g of platinum carbon supported platinum content of from 20 wt.% and 0.07 6 g of 1 M tetrabutyl ammonium hydroxide solution (TBAON) in methanol, and 1.2 g of propylene carbonate (dispersing agent). The fuel cell is collected and tested according to the procedure described in Example 1. The characteristics of the flow field are shown in Figures 3, 4 and 5 in the flow conditions shown in Table 1. As a result of careful study of Example 2a curve is assumed that the apparent weight limit on the curve transfer fluid is not caused by the presence in the flow field, but rather all of the oxygen uptake in the air supplied to the fuel cell. The current limit is available even at 2A / cm 2 when the air stoichiometry IX at 2A / cm 2. Curve shows the possibility of the fuel cell at 2A / cm2 with air stoichiometry only slightly greater than 1. When this fuel cell is set to a multiple battery as part of the power generating system, the ability to operate at such low stoichiometry would contribute to minimize the cost of creating a gas pressure subsystem. Further increasing the flow rate (Examples 2b, 2c) of the smoothed curve with the assumption that the characteristics were almost entirely confined to the resistance element, and further confirmed the absence of mass transfer limitations. The characteristic curve obtained using pure oxygen as the feed gas as shown in Figure 3 as Example 2d.
EXAMPLE 3
MEA / electrode structure was prepared using the procedure described in Example 1, except that two electrode paste as described in Examples 1 and 2 are obtained separately. Example 2 The paste is applied to the glass fibers card designed for use with the cathode side of the membrane, and completely dried, followed by applying a paste as described in Example 1. The paste described in Example 1 was applied to the fiberglass card designed for use with the anode side of the membrane. The platinum content equal to 0.14 mg / cm 2 on the anode side of the membrane and 0.25 mg / cm 2 on the cathode side of the membrane. The fuel cell is collected and tested as described in Example 1. The characteristics of the fuel cell shown in Figures 4 and 5 in the flow conditions shown in the table.
EXAMPLE 4
Membrane-electrode assembly is obtained as follows:
Obtained ion exchange membrane is made from perfluorosulfonic acid ionomer having an equivalent weight (EW) of 800, a thickness of 0.062 mm in a dry state and 0.127 mm in the fully hydrated state, cut into sheets 11 cm by 11 cm and placed into a NaOH bath to convert it into Na + - shape. The electrode paste was prepared by mixing 2.03 g of 3.7 wt% solution of perfluorosulfonic acid ionomer having an equivalent weight (EW) of 770 (50: 50% by volume solution in ethanol: water). 0.1875 g of platinum-on-carbon catalyst with 20% platinum content (supplied by E-TEK, Natick, MA), 0.105 g of tetrabutylammonium hydroxide (TBAON) and 0.6 grams of glycerol. The mixture was stirred overnight stirring before or until the mixture becomes homogeneously dispersed. Then the mixture is introduced an additional 1.2 g of glycerin.
The catalyst paste is applied on clean card 9 cm 2 of glass fibers coated with polytetrafluoroethylene (brand SiEyAr Industries, New Haven, CT), which is dried in an oven at 110 o C. The catalyst ink smeared cards more than two times, and then completely dried before applying the second and third layers. MEA is formed by coupling the coated blank on each side of the ionomer membrane that is dried on a vacuum table. Card and the membrane is placed in a press at 195 o C and pressed at a pressure of 445 N / cm 2 cards for 5 min. Pressing the press is cooled to room temperature before opening. The card is then peeled from the catalyst layer, delivering the film to adhere to the membrane surface. The platinum content of the catalyst bed and the thickness equal to 0.14 mg / cm 2 and 5 um on the anode side the membrane, 0.25 mg / cm 2 and 8 micrometers on the cathode side of the membrane, respectively.
Separate intermediate layers (between the MEA and flow field) of a graphite cloth impregnated with a mixture of carbon and polytetrafluoroethylene particles (available as a Elat from E-TEK, Inc., Natick, MA) are placed adjacent to both active layers in the assembly element and held together by means of gaskets PTFE film and the compression element. Finished units are then tested in a test fuel cell obtained by Fuel Cell Technologies. Inc.. (Santa Fe, New Mexico). the flow fields are composed of solid graphite blocks with machined chopped snake channels.
The element is mounted on a singleton test stand made by Fuel Cell Technologies, Inc. (Santa Fe, New Mexico). The anode (H2) and cathode (air) flows are kept constant and does not vary with the current density. The flow rates for this experience defined by specifying a current density. For example, if the anode flow rate of H2 is 2X stoichiometric at 1.0 A / cm 2, then the flow rate is doubled compared with the rate required to maintain a current density of 1 A / cm 2. Thus, when the cell at 0.5 A / cm 2 is a flow 4X compared with flow required to sustain the current density. The anode and cathode are maintained at a pressure of 138 kPa and 207 respectively. The temperature of the element is equal to 80 o C, while the external humidifiers are set at 100 o C for the anode and at 85 o C for the cathode. Element preconditioned under a load of 0.5 V for 12 hours. The element characteristics are shown in Figure 7. Anode flow rate of H 2 equal to 2X stoichiometric at 1.0 A / cm 2, and the cathode flow speed is 3X stoichiometric at 1.0 A / cm 2.
EXAMPLE 5
The membrane and electrode structures are obtained as follows: (MEA 1):
Obtained ion-exchange membrane made of perfluorosulfonic: ionomer having equivalent weight 800, 60 microns (2.4 mils) dry and 127 microns (5 mils) in a fully hydrated (available from The Dow Chemical Company under the trademark 13204.20 HIS) , is cut into sheets 11 cm by 11 cm and placed into a NaOH bath to convert at -fopmy Na +. The electrode paste was prepared by mixing 1.08 g of a 5.79 wt% solution of the above ionomer (in a 50: 50% by volume solution of ethanol: water)., 0.1875 g of 20% platinum on a carbon support (commercially available from E-TEK (Natick , MA)) and 0.114 g of tetrabutylammonium hydroxide (TBAON), and 0.6 g of propylene carbonate (dispersing agent). The mixture was stirred overnight stirring before or until the mixture becomes homogeneously dispersed. Then the mixture is introduced an additional 1.2 g of propylene carbonate.
The catalyst paste is smeared on a clean, 9 cm2 card fiberglass coated with polytetrafluoroethylene (supplier firm SiEychAr Industries, New Haven, CT), which is dried in an oven at 110 o C and pre-weighed. Card smeared twice a catalyst paste, which is fully dried before applying the second coat. The platinum content equal to 0.14 mg / cm2 on the anode and 0.25 mg / cm2 on the cathode. MEA is formed by conjugation coated blank on each side of the ionomer membrane that is dried on a vacuum table. Card and the membrane are placed between two stainless steel plates with holding them as they are placed in the press. The package is placed in a press at 195 o C and pressed at a pressure of 445 N per 1 cm 2 of the card for 5 min. Pressing the press is cooled to room temperature before opening. The card is peeled from the catalyst layer leaving the film adhered to the surface of the membrane.
Another MEA sample (UME2) is obtained by applying the catalyst ink directly to the surface of the ionomer membrane. The amount of paste applied is determined by weighing the bottle and brush before and after application of the paste. Paste is applied once again in the form of multi-layer coatings, but in this case, satisfactory coatings are applied without requiring complete drying of the paste between applications. The membrane is held in place on a vacuum table having a fine sintered stainless steel frit on top of a heated vacuum manifold board. When the paste is applied, a vacuum table is maintained at a temperature between 45 and 60 o C. The second side of the membrane may be coated in the same manner. The structure is then pressed as described for MEA 1.
The membrane and ionomer binder of both samples again in protonic form are transferred at reflux in 1-normal sulfuric acid for 0.5 hours. The MEA was dried again on the vacuum table and stored in a dry environment until use.
Intermediate layer (PS-1) is obtained as follows:
The paste obtained from 3 g of Vulcan Carbon XC-72, 2 grams of 50 wt.% Solution perftortsiklobutanovogo polymer (poly (1,1,2-tris (4-triftorviniloksifenil) ethane, prepared as described in U.S. Patent 5,037,917, and treated on ![]() -stadii mezitelene to obtain a polymer with an average molecular weight ranging from 4,000 to 8,000) and 31 g of mesitylene. The paste is applied in two application to the untreated graphite paper density of 0.25 g / cm 3, 0.206 mm thick having a porosity of 87% and an average pore size of 50 microns (Spectracorp provider company, Lawrence, MA) to obtain a content of 2 mg / cm 2 polymer and carbon. For the pasta does not require complete drying between coats. The solvent was evaporated and the polymer was fully cured at 200 o C under vacuum for 1 hour. The graphite-polymer layer is placed adjacent to the active layer in the cell assembly and held together by a gasket, coated with polytetrafluoroethylene and the compression element.
-stadii mezitelene to obtain a polymer with an average molecular weight ranging from 4,000 to 8,000) and 31 g of mesitylene. The paste is applied in two application to the untreated graphite paper density of 0.25 g / cm 3, 0.206 mm thick having a porosity of 87% and an average pore size of 50 microns (Spectracorp provider company, Lawrence, MA) to obtain a content of 2 mg / cm 2 polymer and carbon. For the pasta does not require complete drying between coats. The solvent was evaporated and the polymer was fully cured at 200 o C under vacuum for 1 hour. The graphite-polymer layer is placed adjacent to the active layer in the cell assembly and held together by a gasket, coated with polytetrafluoroethylene and the compression element.
Another sample of the intermediate layer / VME (PS2) can be obtained in the following manner:
Solder paste is obtained by mixing carbon powder Vulcan XC-72R with 2 wt. % Dispersant (grade Triton X-100, supplier DuPont) to give a solution containing 20% solids, which was stirred overnight. . Then, polytetrafluoroethylene latex (PTFE) (brand T-3OV, supplier DuPont) diluted to a content of 6 wt% solids, is added in an amount sufficient to provide a weight ratio of carbon black: PTFE of 3: 1 and gently mixed for 1- 2 minutes. The paste is applied to the untreated graphite paper 0.23 mm thick, having a density of 0.25 g / cm 3, a porosity of 87% and an average pore size of 50 microns (obtained from Spectracorp firm) using Meyer Rod 40 and air dried. The sample was then placed in an oven with an inert atmosphere at 340 o C to sinter the PTFE to make it hydrophobic. This way it turns the coating weight of 2 mg / cm 2 (solid carbon and PTFE).
Nodes membrane-electrode are then tested in a test fuel cell manufactured by Fuel Cell Technologies, Inc. (Santa Fe, New Mexico). the flow fields are composed of solid graphite blocks with machined slotted serpentine channels. VME element is set with an intermediate layer on each side. Element is set to the singleton test stand made by Fuel Cell Technologies, Inc., The anode (H2) and cathode (air or O 2) flows are kept constant and does not vary with the current density. The flow rates used for this experiment, determined by specifying the current density and the stoichiometric factor for this current density. For example, cathode air flow rate can be defined as 2x stoichiometric at 1.0 A / cm 2. In this case, the flow rate is double compared to the speed required to maintain a current density of 1 A / cm 2. Thus, when the cell at 0.5 A / cm2, this same flow is four-fold compared to that required to maintain the current density. The anode and cathode are maintained at a pressure of 207 kPa and 276 respectively. Cell temperature was 80 o C, while the external humidifiers are kept at 100 o C for the anode and 85 o C for the cathode. Element preconditioned under a load of 0.5 V for 12 hours.
9 shows the characteristics of the fuel cell containing MEA 1 and PS1 obtained as described above. The figure shows that the fuel cell performance utilizing air as a fuel, to approach the performance of oxygen. Hydrogen flow rate observed same stoichiometry as the air or oxygen flow, and the gas pressure on the anode and the cathode is equal to 207 and 276 kPa, respectively.
10 shows the characteristics of the same fuel cell at a hydrogen pressure of 207 kPa at a flow rate of 2X at 1.0 A / cm 2 and an air pressure of 276 kPa at a flow rate of 3X at 1.0 A / cm 2 for the entire curve.
11 illustrates operation of the fuel cell containing MEA 1 and MS 2 obtained as described above, under the same flow conditions as used in the example shown in Figure 10.
EXAMPLE 6
VME is obtained as described in Example 5 (MEA 1). The paste used to prepare the small pore region was prepared as described in Example 51 (PS1), except that it is applied to a graphite paper of thickness 225 microns (9.0 mils) with a porosity of 84.5%, obtained from Toray ( Tokyo, Japan). Macroporous graphite paper is functioning as both part of the intermediate layer and the flow field as a singleton test bench. The outer ends of graphite paper are filled with an inert material to prevent release of the reactant gases. In these tests, a single element reactant gases are supplied to the surface of the graphite paper in such a way that the flow then 'takes place in the plane of the graphite paper in the direction parallel to the plane of the active catalyst layer. Gases are fed to the porous graphite paper using pyrolyzed graphite blocks with a single feed and discharge channel (supplier firm PiOuSiOu Graphite, Decatur, TX). Figure 12 shows the characteristics of the MEA described above (for Figure 11) at hydrogen and air flow at a stoichiometry of 2 at 2.0 A / cm 2 hydrogen pressure at 138 kPa and 207 kPa air pressure for the entire curve.
EXAMPLE 7
VME is obtained as described in Example 6 (MEA 1). Graphite fibers and carbon powder used to prepare the finely porous layer impregnated by soaking them in a 1 wt.% Toluene solution of tridecafluoro-1,1,2,2-tetragidrooktil-1-trichlorosilane at 80 o C for 3 minutes to make them hydrophobic. The fibers and particles were then rinsed with excess toluene and dried at room temperature. The graphite fiber obtained length of 6.85 mm. The fibers are mixed in a blender in a glycerol mixture Uorinta for 10 minutes or until until a desired length (less than 1 mm). The fibers are rinsed with excess water and dried. Fine-porous layer is produced by spreading a second paste on top of the catalyst bed. The paste was prepared by mixing 0.05 g of the treated graphite fibers (7 microns in diameter, the supplier company Fortafil), 0.05 g of carbon powder (Vulcan XC-grade 72), 1.0 g of 5 wt.% Nafion solution, 0.07 g TBAON (1 molar solution in methanol) and 1.2 g of propylene carbonate.
MEA coated with a finely porous layer is set to a single element with a porous experienced graphite flow field (0.2 g / cm 3, 600 microns (24 mils) thick graphite paper from Spectracorp company having a porosity of about 90%) and tested in accordance with procedure described in example 6.
FIG. 12 shows the characteristics when the above-described MEA flows of hydrogen and air at stoichiometry 2 at 2.0 A / cm 2, at 138 kPa hydrogen pressure at 207 kPa air pressure for the entire curve.
EXAMPLE 8
A membrane - electrode is obtained as follows:
Obtained ion exchange membrane is made from perfluorosulfonic acid ionomer having an equivalent weight (EW) of 800, a thickness of 60 microns (2.4 mils) dry and 127 microns (5 mils) in the fully hydrated state (supplier company Dow Chemical Company) is cut into sheets 11 x 11 cm and placed into a NaOH bath to convert it into Na + -form. The electrode paste was prepared by mixing 1.08 g of a 5.79 wt% solution of the ionomer (in a 50:50 vol% ethanol solution:. Water)., 0.1875 grams of a 20 wt% platinum on carbon (supplier company E-TEK, Natick,. MN), 0.114 g of tetrabutylammonium hydroxide (TBAON) and 0.6 g of propylene carbonate. The mixture was stirred overnight stirring before or until the mixture becomes homogeneously dispersed. Then the mixture is introduced an additional 1.2 g of propylene carbonate.
The catalyst paste is spread on the net 9 cm 2 coated fiberglass cards polytetrafluoroethylene (supplier firm SiEychAr Industries, New Haven, CT), which is dried in an oven at 110 o C and pre-weighed. Cards smeared twice a catalyst paste, which is fully dried before applying the second coat. MEA is formed by conjugation coated blank on each side of the ionomer membrane that is dried on a vacuum table. Card and the membrane is placed in a press at 1,95 o C and pressed at a pressure of 445 N per 1 cm 2 of the card during 5 min. Pressing the press is cooled to room temperature before opening. The card is peeled from the catalyst layer leaving the film on the surface of the membrane. The platinum content of the catalyst bed and the thickness equal to 0.14 mg / cm 2 and 10 m on the anode side the membrane, 0.25 mg / cm 2 and 17 microns on the cathode side of the membrane, respectively.
Separate intermediate layers (between the MEA and flow field) of a graphite cloth impregnated with a mixture of carbon particles and polytetrafluoroethylene (available under the trademark Elat from E-TEK, Inc., Natick, MA) are placed adjacent to both active layers in the assembly element and held together using coated with polytetrafluoroethylene gaskets and compression element. The resulting assemblies are then tested in a test fuel cell manufactured by Fuel Cell Technologies, Inc. (Santa Fe, New Mexico). the flow fields are composed of solid graphite blocks with machined channels carved snake.
The element is mounted on a singleton test stand made by Fuel Cell Technologies, Inc.. (Santa Fe, New Mexico). The anode (H2) and cathode (air) flows are kept constant and does not vary with the current density. The flow rates for this experience defined by specifying a current density. For example, if the anode flow rate of H2 is equal to 2X stoichiometric at 1.0 A / cm 2, then the flow rate is a two-time flow rate that is required to maintain a current density of 1 A / cm 2. Thus, when the cell at 0.5 A / cm 2, the same current is 4x to that required to sustain the current density. The anode and cathode are maintained at a pressure of 207 kPa and 176 respectively. Cell temperature was 80 o C while the external humidifiers are set at 100 o C for anode and at 85 o C for the cathode member is pre-load is maintained at 0.5 V for 12 hours. The element characteristics are shown in Figure 13. Anode flow rate of H 2 equal to 2X stoichiometric at 1.0 A / cm 2, and the cathode flow rate of air equal 3X stoichiometric at 1.0 A / cm 2.
EXAMPLE 9
A membrane - electrode obtained as described in Example 8, except that TBAON not introduced into the catalyst paste. The content of platinum and the thickness of the catalyst layer on the anode and cathode side of the membrane is equal to 0.15 mg / cm 2 and 10 micron, and 0.25 mg / cm 2 and 16 microns, respectively.
The intermediate layers are obtained as follows:
The paste obtained from 3 g of Vulcan Carbon XC-72, 2 grams of 50 wt.% Solution perftortsiklobutanovogo polymer (poly (1,1,2-tris (4-triftorviniloksifenil) ethane), prepared as described in U.S. Patent 5,037,917 and processed in ![]() -stadii in mesitylene to produce a polymer with an average molecular weight ranging from 4,000 to 8,000) and 31 g of mesitylene. The paste is applied in two application to the untreated graphite paper 0.206 mm thick having a density of 0.25 g / cm 3, a porosity of 87% and an average pore size of 50 microns (Spectracorp provider company, Lawrence, MA), c obtaining content 2 mg / cm 2 polymer and carbon. For the pasta does not require complete drying between coats. The solvent was evaporated and the polymer was fully cured at 200 o C under vacuum for 1 h. Grafitopolimerny layer located adjacent to the active layer in the assembly element and held together by a polytetrafluoroethylene-coated gasket and the cell compression.
-stadii in mesitylene to produce a polymer with an average molecular weight ranging from 4,000 to 8,000) and 31 g of mesitylene. The paste is applied in two application to the untreated graphite paper 0.206 mm thick having a density of 0.25 g / cm 3, a porosity of 87% and an average pore size of 50 microns (Spectracorp provider company, Lawrence, MA), c obtaining content 2 mg / cm 2 polymer and carbon. For the pasta does not require complete drying between coats. The solvent was evaporated and the polymer was fully cured at 200 o C under vacuum for 1 h. Grafitopolimerny layer located adjacent to the active layer in the assembly element and held together by a polytetrafluoroethylene-coated gasket and the cell compression.
MEA and intermediate layers were assembled together and tested in a fuel cell as described in Example 8. Although the element preconditioned for 12 hours, its peak performance is achieved only after 1 hour. The characteristic curve is shown in Figure 14. The flow rates are the same as in Example 8.
CLAIM
1. An electrochemical fuel cell having a membrane - electrode and adjacent to a layer of electrically conductive porous material having a porosity of at least 50% and an average pore size of at least 35 microns.
2. The fuel cell according to Claim. 1 wherein the porous conductive material has a thickness of not less than 0.5 mm of the material and size 76.2 x 76.2 mm can absorb at least 0.5 g of water per 1 gram of material over 10 on standing vertically in the water at a depth of 4.76 mm.
3. The fuel cell according to claim. 2, wherein the porous conductive material has a thickness of not less than 0.5 mm and can absorb at least 1 gram of water per 1 g of the material.
4. The fuel cell according to Claim. 1, wherein the layer of electrically conductive porous material has a porosity of at least 75%.
5. The fuel cell according to Claim. 1, wherein the layer of electrically conductive porous material has a porosity of at least 80%.
6. The fuel cell according to Claim. 1, wherein the layer of electrically conductive porous material having an average pore size of at least 45 microns.
7. The fuel cell according to Claim. 1, wherein the layer of electrically conductive porous material having an average pore size of at least 50 microns.
8. The fuel cell according to Claim. 1, wherein the layer of electrically conductive porous material having an average pore size of not more than 250 microns.
9. Fuel element according to Clause 1, wherein a membrane - electrode comprises a solid polymer membrane having at least two layers of catalytically active particles paste on at least one side thereof, wherein at least two layers of pasta catalytically active particles contain polymers polytetrafluoroethylene having pendant sulfonic acid groups, the equivalent weight of which differs by more than 50 and wherein the layer having the highest equivalent weight is positioned adjacent to a layer of porous electrically conductive material.
10. The fuel cell of claim. 9, wherein the porous material has a porosity of at least 80% and an average pore size of at least 50 microns.
11. The fuel cell of claim. 10 wherein the porous material has a thickness of not less than 0.5 mm of the material and size 76.2 x 76.2 mm can absorb at least 1 gram of water per 1 g of the material 10 when exposed to a vertically in water at a depth of 4.76 mm.
12. A fuel cell comprising at least three fuel cell according to claim. 1, arranged in series.
13. Node membrane - electrode having an ion-exchange membrane and at least two active layers positioned on the same side of the membrane and at least two active layers positioned on the same side of the membrane, wherein the active layers comprise catalytically active particles and ionomer average equivalent weight ionomers in the layers differ by at least 50 and the active layer positioned closest to the membrane contains the ionomer with the lower average equivalent weight.
14. The assembly of claim. 13 wherein at least one of the active layers comprises at least 99 wt. % Of a mixture of catalytically active particles and the ionomer.
15. The assembly of claim. 13 wherein the two active layers together have a thickness of not less than 1 micron.
16. The assembly of claim. 13 wherein the two active layers together have a thickness of not less than 5 microns.
17. The assembly of claim. 13 wherein the two active layers together have a thickness of not less than 10 microns.
18. The assembly of claim. 13 wherein the two active layers together have a thickness of not more than 30 microns.
19. The assembly of claim. 13 wherein at least one of the active layers has a porosity of at least 30%.
20. The assembly of claim. 13 wherein at least one of the active layers has a porosity of at least 50%.
21. The assembly of claim. 13 wherein the at least one active layer has an average pore size ranging from 0.01 to 10.0 microns.
22. The assembly of claim. 13 wherein the at least one active layer has an average pore size ranging from 0.03 to 0.5 microns.
23. The assembly of claim. 13 wherein the catalytically active particles are present in an amount sufficient to provide the level of content on the cathode side of the membrane in the range of 0.05 to 0.45 mg / cm 2 and at the level of the anode side of the membrane in the range of 0.01 to 0.15 mg / cm 2.
24. Node membrane - electrode having an ion-exchange membrane and at least one active layer positioned on one side of the membrane, wherein the active layers contain (a) catalytically active particles, and (c) an ionomer having an equivalent weight in the range from 650 to 950 and which is substantially insoluble in water at a temperature less than 100 o C.
25. The assembly of claim. 24 wherein the active layer comprises at least 99 wt. % Of a mixture of catalytically active particles and the ionomer.
26. The assembly of claim. 24 wherein the active layer has a thickness of not less than 1 micron.
27. The assembly of claim. 24 wherein the active layer has a thickness of not less than 5 microns.
28. The assembly of claim. 24 wherein the active layer has a thickness of not less than 10 microns.
29. The assembly of claim. 24 wherein the active layer has a thickness of not more than 30 microns.
30. The assembly of claim. 24 wherein the active layer has a porosity of at least 30%.
31. The assembly of claim. 24 wherein the active layer has a porosity of at least 50%.
32. The assembly of claim. 24 wherein the active layer has an average pore size ranging from 0.01 to 10.0 microns.
33. The assembly of claim. 24 wherein the active layer has an average pore size ranging from 0.03 to 0.5 microns.
34. The assembly of claim. 24 wherein the catalytically active particles are present in an amount sufficient to provide the level of content on the cathode side of the membrane in the range of 0.05 to 0.45 mg / cm 2 and at the level of the anode side of the membrane in the range of 0.01 to 0.15 mg / cm 2.
35. A composition comprising (a) catalytically active particles, (c) an organic compound, and (c) an ionomer having an equivalent weight ranging from 650 to 950, and which is substantially insoluble in water at a temperature less than 100 o C.
36. An electrochemical fuel cell having a membrane - electrode and adjacent to a layer of electrically conductive porous material having at least two portions with different mean pore sizes, wherein a first portion of the layer adjacent to the membrane - electrode has a porosity not porosity exceeding a second portion of the layer adjacent to the opposite side of the layer; the second portion has a porosity of at least 82% and the second portion has an average pore size that is not less than 10 m and not less than 10 times the average pore size of the first part.
37. The fuel cell of claim. 36 wherein the first portion of the porous layer is hydrophobic.
38. The fuel cell of claim. 36 wherein the first portion of the porous layer comprises a polytetrafluoroethylene polymer.
39. The fuel cell of claim. 36 wherein the first portion of the porous layer contains a copolymer perftoralkilakrilovy.
40. The fuel cell of claim. 36 wherein the first portion of the porous layer comprises a polymer perftortsiklobutanovy.
41. The fuel cell of claim. 36 wherein the average pore size of the first portion of the porous layer is at least 0.1 microns.
42. The fuel cell of claim. 36 wherein the average pore size of the first portion of the porous layer is at least 1 micron.
43. The fuel cell of claim. 36 wherein the porosity of the second portion of the porous layer is not less than 82.5%.
44. The fuel cell of claim. 36 wherein the porosity of the second portion of the porous layer on its second side is at least 85%.
45. The fuel cell of claim. 36 wherein the porosity of the second portion of the porous layer is not less than 87.5%.
46. The fuel cell of claim. 36 wherein the second portion of the porous layer is more hydrophilic than the first portion.
47. Топливный элемент по п. 36, в котором первая часть пористого слоя имеет не менее 30 мкм.
48. Электрохимический топливный элемент, имеющий узел мембрана - электрод со смежным с ним нетканым пористым слоем электропроводящего пористого материала, который имеет по крайней мере две части с различными средними размерами пор, где первая часть слоя, смежная с узлом мембрана - электрод, имеет пористость, не превышающую пористость второй части слоя, смежной с противоположной стороной слоя; вторая часть имеет пористость не менее 50% и вторая часть имеет средний размер пор, который составляет не менее 35 мкм и который не менее чем в 10 раз превышает средний размер пор первой части.
49. Топливный элемент по п. 48, в котором первая часть пористого слоя является гидрофобной.
50. Топливный элемент по п. 48, в котором первая часть пористого слоя содержит политетрафторэтиленовый полимер.
51. Топливный элемент по п. 48, в котором первая часть пористого слоя содержит перфторалкилакриловый сополимер.
52. Топливный элемент по п. 48, в котором первая часть пористого слоя содержит перфторциклобутановый полимер.
53. A process for preparing an electrochemical fuel cell having a membrane - electrode, comprising the steps of: (a) applying a layer of a conductive composition to a sheet of porous conductive material having a porosity of at least 82%, under conditions sufficient to form a porous solid layer conductive composition on one the sheet of porous conductive material, thereby forming a composite, and (c) placing the composite adjacent to the membrane - electrode so that the side of the composite to which the conductive composition was applied, it faces the said assembly.
54. The method of claim. 53 wherein the conductive polymer composition comprises polytetrafluoroethylene, perftoralkilakrilovy perftortsiklobutanovy polymer or copolymer.
. 55. The method of claim 54, wherein the conductive composition additionally comprises carbon fibers and / or powders and the weight ratio of the fibers and / or powders of polymers and is at least 1: 1.
. 56. The method of claim 54, wherein the conductive composition additionally comprises carbon fibers and / or powders and the weight ratio of the fibers and / or powders of polymers and is at least 3: 1.
. 57. The method of claim 54, wherein the conductive composition additionally comprises carbon fibers and / or powders and the weight ratio of the fibers and / or powders of the polymers and is not more than 10: 1.
. 58. The method of claim 54, wherein the conductive composition additionally comprises carbon fibers and / or powders and the weight ratio of the fibers and / or powders of the polymers and is not more than 5: 1.
59. A composition comprising (a) catalytically active particles, (c) an organic compound having a pK of at least 18 and basicity parameter ![]() less than 0.66, and (c) a polymeric binder.
less than 0.66, and (c) a polymeric binder.
60. The composition of claim. 59 wherein the polymeric binder is a proton form of an ionomer.
61. The composition of claim. 59 wherein the polymeric binder has an equivalent weight ranging from 600 to 1,200.
62. The composition of claim. 59 wherein the polymeric binder has an equivalent weight ranging from 700 to 950.
63. A composition comprising (a) catalytically active particles, (c) an organic compound selected from ethylene carbonate, propylene carbonate, butylene carbonate, etilenkarbamata, propilenkarbamata, butilenkarbamata, acetone, acetonitrile, difluorobenzene, and sulfolane, and (c) a polymeric binder.
64. A composition comprising (a) catalytically active particles, (b) and propylene carbonate (c) a polymeric binder.
65. A method of producing the membrane assembly - electrode which comprises the sequential steps of (i) applying a composition layer according to claim 59 to a solid polymer electrolyte, carbon fiber paper, or a release substrate;. (Ii) heating the composition under conditions sufficient to volatilize at least 95% of the organic compound having a pK of at least 18 and basicity parameter ![]() less than 0.66; and (iii) placing the composition in contact with a solid polymer electrolyte, if the composition was not applied directly to the solid polymer electrolyte, thereby forming a membrane assembly - electrode.
less than 0.66; and (iii) placing the composition in contact with a solid polymer electrolyte, if the composition was not applied directly to the solid polymer electrolyte, thereby forming a membrane assembly - electrode.
66. The method of claim. 65 wherein the composition is applied in an amount sufficient to provide a layer of the composition, which after drying has a thickness of protonation of less than 10 microns.
67. The method of claim. 65 wherein the composition is applied in an amount sufficient to provide a layer of the composition, which after drying has a thickness of protonation of less than 15 microns.
68. The method of claim. 66 wherein the porosity of the dried and protonated layer is in the range of from 30 to 90%.
69. The method of claim. 66 wherein the porosity of the dried and protonated layer is in the range of from 50 to 90%.
70. The method of claim. 66 wherein the average pore size of the dried and protonated layer is in the range of 0.03 to 0.5 microns.
71. A method of producing the membrane assembly - electrode which comprises the sequential steps of (i) applying a layer of the composition of claim 64 to a solid polymer electrolyte, carbon fiber paper, or a release substrate;. (Ii) heating the composition under conditions sufficient to volatilize at least 95% of an organic compound selected from ethylene carbonate, propylene carbonate, butylene carbonate, etilenkarbamata, propilenkarbamata, butilenkarbamata, acetone, acetonitrile, difluorobenzene, and sulfolane, and (iii) placing the composition in contact with a solid polymer electrolyte, if the composition was not applied directly to the solid polymer electrolyte, thereby forming a membrane assembly - electrode.
72. A method of producing the membrane assembly - electrode which comprises the sequential steps of (i) applying a layer of the composition of claim 64 to a solid polymer electrolyte, carbon fiber paper, or a release substrate;. (Ii) heating the composition under conditions sufficient to volatilize at least 95% of propylene; and (iii) placing the composition in contact with a solid polymer electrolyte, if the composition was not applied directly to the solid polymer electrolyte, thereby forming a membrane assembly - electrolyte.
73. The method of claim. 72 wherein the composition is applied in an amount sufficient to provide a layer of the composition, which after drying has a thickness of protonation of less than 10 microns.
74. The method of claim. 72 wherein the composition is applied in an amount sufficient to provide a layer of the composition, which after drying has a thickness of protonation of less than 15 microns.
75. The method of claim. 73 wherein the porosity of the dried and protonated layer is in the range of from 30 to 90%.
76. The method of claim. 73 wherein the porosity of the dried and protonated layer is in the range of from 50 to 90%.
77. The method of claim. 73 wherein the average pore size of the catalyst bed is in the range of 0.03 to 0.5 microns.
78. The method of claim. 72 wherein the at least two different compositions of claim. 6 are applied sequentially to the electrolyte, paper, or substrate, the compositions comprise polytetrafluoroethylene polymers having pendant sulfonic acid groups, as binders, the average weight of which differ at least than 50 for each composition, and wherein the composition has a binder with the lowest average equivalent weight, is located adjacent to the solid polymer electrolyte.
print version
Publication date 06.02.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.