| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2277274
![]()
METHOD OF CHEMICAL ENERGY TRANSFORMATION
IN ENERGY OF VARIABLE ELECTRIC CURRENT
The name of the inventor: Deremyan Albert Amaikovich (RU); Petrov Sergey Borisovich (RU)
The name of the patent owner: Deremyan Albert Amaikovich (RU); Petrov Sergey Borisovich (RU)
Address for correspondence: 344010, Rostov-on-Don, st. Chekhov, 103/271, ap. 32, A.A. Derement
Date of commencement of the patent: 2004.11.10
The invention relates to the field of direct conversion of chemical energy into electrical energy and can be used in current sources. According to the invention, a method for producing AC electric power comprises forming a symmetrical electrochemical pair of gas permeable inert electrodes separated by an electrolyte layer (ion exchange membrane), each of which alternately operates with a fuel and oxidizing electrode. The technical result of the invention is to increase the efficiency of the conversion process and increase the resource.
DESCRIPTION OF THE INVENTION
The invention relates to the field of direct conversion of chemical energy into electrical energy and can be used in current sources.
A method is known for converting the chemical energy of matter into electrical energy (NV Korovin "Electrochemical generators", Izd-vo Energia, Moscow, 1974, pp. 5-12), in which a pair of reactive electrodes separated by an electrolyte layer is fed Reagents and withdraw the reaction products.
The disadvantages of the known method are as follows.
The use of a conductor with ionic conductivity is associated with the transfer of the substance of the electrolyte, its destruction and polarization of the electrodes. Another, no less significant disadvantage is the use of an oxidizer and a reductant of high chemical purity, since even minor impurities contaminate the fuel cell, reduce efficiency and shorten its useful life.
The basis of the claimed invention is the task of creating a method for converting the energy of a chemical substance into electrical energy, which ensures an increase in the efficiency of the process.
The technical result that can be obtained by implementing the claimed method consists in increasing the efficiency of the conversion process, significantly increasing the life of the fuel cell (fuel cell), reducing the cost of fuel and oxidizing agent, in obtaining directly into the fuel cell an alternating current with the possibility of its further transformation.
The essence of the proposed method for converting the chemical energy of fuel into electrical energy is as follows:
They form an electrochemical pair of gas-permeable inert electrodes made of a material with electronic conductivity separated by an electrolyte layer, an oxidizer chamber and a reducing agent, switching compounds, a functional support system, and supplying reagents to the electrochemical reaction zones, diverting the combustion products, alternately swapping the cathode and anode in As a result, the polarization of the electrodes accelerates the course of the electrochemical process without adversely affecting it, and the electrolyte ions perform vibrational movements in the electrolyte volume that are not accompanied by the transfer of the electrolyte substance.
 |
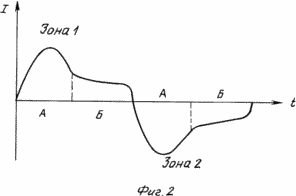 |
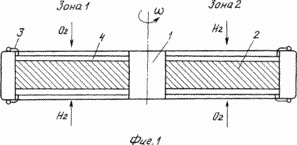
The method is explained in the drawing, where FIG. 1 is a schematic diagram of an apparatus for providing polarity reversal of electrodes, and FIG. 2 is a graph of current variation. FIG.
The processes of adsorption of reagents and reactions of electrochemical combustion on TE electrodes made of a material with electronic conductivity, capable of decomposition and ionization of both fuel and oxidizer in equal measure, closed by an electrical circuit to an external load, lead to the appearance of an electric current in the circuit, accompanied by a decrease The strength of the electric field between the electrodes. When changing the reagents on the electrodes (or changing the electrodes of the chambers), a similar process takes place, but with the direction of the electric current reversed. And the electrolyte works infinitely long, since the ions of the electrolyte make vibrational movements in the volume of the electrolyte, which is not accompanied by the transfer of matter.
The construction and materials of a symmetrical electrochemical pair comprising two inert gas permeable electrodes separated by an electrolyte layer (ion exchange membrane), switching compounds, functional support systems are selected in such a way that the deviations of the maximum amplitude value of the current in counterphase operations of the generator are equal in magnitude or differed More than 25%.
The circuit of the device for ensuring polarity reversal of the electrodes is performed, for example, on a rotating rotor (FIG. 1).
Hydrogen, methane and other gaseous hydrocarbons, vapors of alcohols or liquid hydrocarbon fuels, incomplete combustion or gas conversion products and the like can be used as the fuel, and oxygen, air, hydrogen peroxide and other oxidants are used as the oxidizing agent.
An example of implementing the method:
The proposed method is carried out by means of the device depicted in FIG.
The rotor 1 of the electrochemical generator (ECG) made of a dielectric material and having an axis of rotation consists of separate symmetrical fuel cells, grouped in groups of phases by switching connections with the possibility of pick-up in each group, each of which consists of a plate of solid electrolyte 2 with deposited The current leads 3 and platinum electrodes 4 deposited on it from both sides.
The ECG stator consists of oxidizer and reducing agent chambers (in zones by the number of phases), stabilization zones and the functional support system.
When the reagents are fed to the electrodes in zone 1, the electrochemical combustion reaction proceeds with the pickup of the most active component (Fig. 2) (zone 1A) and with the stabilization of the reaction products with the open external circuit (zone 1B). When this group of electrodes enters zone 2, an analogous process takes place, but with a change in the direction of motion of the electric current to the opposite one.
The advantage of the claimed method is the possibility of obtaining an alternating electric current directly in the FC, a significant increase in the service life and the use of low chemical purity reagents.
CLAIM
A method for converting the energy of a fuel and an oxidizer into energy of an alternating electric current in an electrochemical generator consisting of a rotor and a stator in which symmetrical fuel cells, each consisting of a solid electrolyte with current leads and electrodes, are grouped and placed on the rotor, and on the stator The oxidizer and reducing agent chambers are placed, the functional support system, characterized in that each electrode is made of an active material capable of decomposing and ionizing both the fuel and the oxidant, and the electrodes alternately perform the functions of the fuel and oxidizing electrodes when the reagents are changed at the electrodes due to their Rotation.
print version
Publication date 16.02.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.