| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2295178
![]()
SOLID SECONDARY SOURCE OF CURRENT
The name of the inventor: Potanin Alexander Arkadevich
The name of the patent holder: Limited Liability Company "High-Energy Battery Systems" (LLC "VEBS")
Address for correspondence: 607189, Nizhny Novgorod Region, Sarov, ul. Silkin, 5, sq. 40, LLC "VEBS", A.A. Potanin
Date of commencement of the patent: 2005.04.21
The invention relates to the field of electrical engineering, namely to secondary electrochemical current sources (batteries). According to the invention, the solid state secondary current source consists of an anode in the form of a metal or metal alloy, the fluorination of which leads to the formation of fluoride or fluorides with a high isobaric formation potential, an electrolyte in the form of a solid fluorine ion conductor with high ionic and low electron conductivity and a cathode in the form of Fluoride or a solid solution of fluorides with a low isobaric formation potential, wherein the anode and the cathode are reversible with respect to fluoride ions at voltages below the decomposition voltage of the solid electrolyte, and the anode, electrolyte and cathode contain at least one component preventing the destruction of the solid- Charge-discharge cycles. The technical result of the invention is to increase the specific energy characteristics of secondary batteries and the long-term preservation of electrical energy.
DESCRIPTION OF THE INVENTION
The invention relates to the field of electrical engineering, namely to secondary electrochemical current sources (batteries), the primary use areas of which are electronic and microelectronic devices in telecommunication systems and laptop computers, electric vehicles and other equipment requiring high-energy and safe secondary electrochemical current sources Batteries) with low self-discharge.
The following parameters of secondary batteries can be considered a promising level for these wide applications:
- Specific energy consumption of 500 W · h / kg,
- The density of electrical energy is 600 W · h / l,
- The number of charge / discharge cycles is about 1000,
- Self-discharge is 1-3% per year.
To achieve the highest specific energy characteristics of electrochemical current sources, the current-forming reaction with the most electropositive cation Li + and / or with the most electronegative anion F- is the most favorable.
For electrochemical sources with high specific energy characteristics, the problem of the safety of current sources is becoming topical. According to (1), the level of specific energy of prospective chemical sources of current of 500-1000 W · h / dm 3 or 1.8-3.6 kJ / cm 3 is already comparable with the level of explosive explosive energy, for example TNT (6,7 KJ / cm 3 ). In this regard, in the group of electrochemical sources with high specific energy characteristics, the most promising are solid-state current sources, in which the anode, electrolyte and cathode are solid substances and a solid-phase current-generating reaction is realized with stable solid-state cathodes and anode both in the charge and discharge process. In this direction, the most energy-intensive and safe ones are solid-state fluorine sources of current based on solid ionic fluoride ions / 1 /. For a wide group of fluorides and solid solutions of fluorides with doping additives, a high mobility of fluoride ions and a correspondingly high fluorine-ion conductivity in the solid phase are characteristic. In this case, the correlation between the anion mobility and the parameters of the crystal lattice is observed / 2 /.
Divalent compounds of the MF 2 type (M = Ca, Sr, Ba, Cd and Pb) with a cation coordination number of 8 are fluorites.
Divalent compounds of the MF 2 type (M = Mg, Mn and Zn) of the compound with the coordination number of 6-rutile.
Trivalent compounds MF 2 , where M = Al, Se and In; M = Y, Gd and Bi; M = La belong to deformed ReO 3 type, YF 3 type and structures of tysonite type, respectively, with coordination cation numbers 6, 9 and 11 respectively.
Lithium fluoride has the structure of NaCl with hexagonal coordination. Zirconium tetrofluoride and ThF 4 have a coordination cation number of 8, and belong to the structure of ZrO 4 .
Fluorite and tisonite structures with cationic coordination numbers 8, 9 and 11 have a higher mobility of fluorine ions, and an increase in anionic conductivity is observed with increasing cationic polarizability, and with an increase in the cationic radius, a decrease.
Using these or other solid fluorine-ion conductors, a wide group of patented current sources is known.
Solid-state current sources based on solid fluorine ion conductors are known, for which charging and discharge processes are possible, that is, to some extent they can be attributed to secondary sources of current. In particular, in / 3 / proposed sources of current, which in the discharged state is the following composition:
- C / PbF 2 (with the addition of KF) / Ag,
- Pb / PbF 2 (with the addition of KF) / Ag,
- Pb / PbF 2 (with the addition of KF) / Cu,
- C / PbF 2 (with the addition of KF) / Cu,
- C / PbF 2 (with the addition of KF) / C,
And in the charged state the following:
- Pb / PbF 2 (with the addition of KF) / AgF / Ag,
- Pb / PbF 2 (with the addition of KF) / CuF 2 / Cu,
- Pb / PbF 2 (with the addition of KF) / PbF 2 / C.
In these current sources, the solid electrolyte is a polycrystalline composition consisting of lead fluoride with the addition of potassium fluoride. The use of an electrode pair of lead-silver fluoride in secondary batteries is characterized by the reversibility of electrode processes, which allows it to be used both as a primary and secondary source of current. However, this current source when it is used in the secondary version, that is, in the battery version, is characterized by low energy consumption. This is due to the fact that when the battery is charged, anodic lead is formed as a result of electrolysis of the solid electrolyte, and consisting of lead fluoride, which leads to the destruction of the electrolyte layer. As a consequence, in the secondary source of said device, when carrying out a charge cycle, the realization of a low charging capacitance is possible, and as a result, the current source has a low electric capacity. For example, in a known solid-state chemical source of current (3), the electrical capacity is -0.65 mAh. The increase in the electrical capacity of this device can only be achieved by increasing the dimensions, which is not always permissible and justified, since current sources in this case have very low specific characteristics. Specifically, from the characteristics of the secondary cell battery cell (electric capacity 0.65 mAh, diameter 15 mm, thickness about 1 mm, average density about 8 g / cm 3 and discharge voltage about 1 V) given in (3), the specific energy intensity is 0 , 45 W · h / kg and an electric energy density of 3.6 W · h / l. At the same time, if we turn to the design of real batteries, these parameters are reduced by another 30-50%. Thus, the secondary solid-state batteries proposed in [3] have very low energy parameters. For example, for nickel-cadmium batteries, the level of these parameters is 70 W · h / kg, 120 W · h / l, and for lithium-ion batteries - 130 W · h / kg and 300 W · h / l.
In the current source proposed in [4], it is possible to slightly increase the specific energy characteristics and approach the level of nickel-cadmium batteries. This is achieved by the fact that in a patented current source consisting of a lead-based anode from a cathode containing silver fluoride and a fluorine-ion conducting electrolyte containing rare-earth metal fluoride, for example LaF 3 , an alkali metal fluoride, for example BaF 2 , And alkali metal fluoride, for example KF or LiF, there is a deeper electrolysis of PbF 2 in the anode layer during charge because the electrolyte is more chemically stable and does not decompose under charge voltage. Of the characteristics of a galvanic cell (electrical capacitance 80 mAh, diameter 20 mm, thickness 1 mm, average density about 7 g / cm 3 and discharge voltage about 1 V), indicated in (4), the specific energy of galvanic cells is 35 W · H / kg and 250 W · h / l and accordingly for batteries one should expect 20 W · h / kg and 130 Wh · h / l. These are rather low characteristics for future applications.
These known current sources are characterized by low energy intensity due to low energy consumption of the anodic interaction of fluoride with lead. The theoretical energy capacity of the anodic interaction of lead with fluorine corresponds to 219 A · h / kg of anode or 26.5 A · h / dm 3 of the anode and the current sources are characterized by a low open circuit voltage (NDC) of 1.2-1.3 V.
In addition, in the device of the reduced secondary solid-state current sources, the problems that arise in the anode and cathode structures as well as at the anode / electrolyte and cathode / electrolyte interfaces during charge and discharge processes are not solved. These problems are due to the fact that for the anodic reaction with charge PbF 2 + 2e - <-> 2F - + Pb due to the difference in the density of PbF 2 and Pb, the volume of the solid phase decreases by 37% (with a discharge correspondingly increases), and For the cathode, for example, when Ag + 2F - <AgF 2 + 2e is being charged - the volume of the solid phase increases by 110% (when the discharge is correspondingly reduced). For solid-phase processes, such changes are very critical and can lead, even with several charge-discharge cycles, to the destruction of the current source, so referring them to the group of secondary current sources is to a large extent conditional.
Thus, the foregoing known solid-state current sources, in which both charge and discharge can be realized, have the following drawbacks:
- low specific energy characteristics, which does not allow the use of current sources for such market segments as electronic and microelectronic devices in telecommunication systems and in laptop computers, electric vehicles and other equipment requiring highly energy-consuming and safe secondary electrochemical current sources (batteries) for operation;
- these current sources do not allow the realization of a large number of charge-discharge cycles, since in their device the problem of the mechanical strength of a galvanic cell is not solved when the density of anode and cathode materials changes during solid-phase reactions during charge-discharge cycles.
To increase the specific energy characteristics of solid-state current sources on the basis of solid fluorine-ionic conductors, higher-energy current-generating reactions involving fluoride ions should be used.
The highest energy characteristics for solid-state fluorine-ion batteries are given in / 1 /. These results were obtained experimentally, which corresponds to the criterion of practical realizability of solid-state fluorine-ion current sources with a very high specific energy intensity. The achieved level of specific energy characteristics corresponds to the required level of the claimed secondary solid-state current source, therefore the device of current sources, known from / 1 /, is considered as the closest and chosen as a prototype.
A known solid-state current source device is as follows: 1 /:
1) solid-state fluorine-ion galvanic cell, which is a ceramic multilayer structure and consists of a solid anode, an electrolyte and a cathode;
2) solid anode - based on metal or alloy, the fluorination of which leads to the formation of fluoride with a high isobaric formation potential and high fluorine-ionic conductivity;
3) solid cathode - heat-resistant metal fluoride or a solid solution of fluorides with high fluorine-ion conductivity and low isobaric formation potential;
4) solid electrolyte - heat-resistant metal fluoride or a solid solution of fluorides with high fluorine-ion conductivity and low electronic conductivity.
Such a device of a solid-state current source makes it possible to realize, with the discharge of a current source, a solid-phase high-energy current-generating reaction involving fluoride ions. When the external circuit is closed, the F ions diffuse through the solid phase of the solid ionic conductor, which forms the base of the cathode, then along the solid electrolyte. Subsequent solid-phase interaction of the fluorine ion with the anode metal leads to the formation of fluoride with a high anionic mobility and the transition of electrons to the external circuit. Thus, during the discharge, the metal anode region adjacent to the electrolyte layer is fluorinated to form a solid ionic conductor and does not block the further discharge process.
As a concrete implementation of such a device of such a current source, the following device designs are known. Solid solutions of fluorides LaF 3 -BaF 2 and CeF 3 -SrF 2 with a content of BaF 2 and SzF 2 of about 6% (mol) were used as the electrolyte of the solid-state fluorine-ion element.
In this case, the current source device has the form (anode / electrolyte / cathode):
- La / LaF 3 -BaF 2 / BiF 3 -KF,
- La / LaF 3 -BaF 2 / PbF 2 -KF,
- Ce / CeF 3 -SrF 2 / BiF 3 -KF,
- Ce / CeF 3 -SrF 2 / PbF 2 -KF.
When discharging an electrochemical current source such as La / LaF 3 -BaF 2 / BiF 3 -KF, the following reactions occur:
At the anode: La + 3F - ---> LaF 3 + 3e -
At the cathode: BiF 3 + 3e - -> Bi + 3F - .
In the case of using PbF 2 -KF in the cathode, the following basic cathodic reaction takes place:
![]()
The realization of such chemical transformations is confirmed by the correspondence of the thermodynamic design values of the EMF and the experimental values of the voltage of the open circuit of the current source.
The specific energy intensity of such current sources is increased when a number of metal oxides are introduced into the cathode on the basis of BiF 3 or PbF 2 solid solutions: CuO, V 2 O 5 , MnO 3 , Ag 2 O, PbO 2 [5, 6]. In this case, when the current source is discharged in the cathode layer, an additional exothermic oxidation-reduction reaction is realized with the formation of solid-phase products.
In particular, at the anode and cathode:
At the anode: 2La + 6F - -6e - -> 2LaF 3 ,
At the cathode: ![]()
The total reaction, which determines the EMF of the current source, has the form:
![]()
Specific energy characteristics of known current sources in the form of a single galvanic cell are given in Table 1.
| Table 1 The energy characteristics of the chemical source of the La / LaF 3 -BaF 2 / BiF 3 -KF current introduced into the cathode of CuO (Discharge temperature 550 ° C, i = 100 mA / cm 2 , operating voltage up to 2 V) | ||||
| Content in cathode CuO,% (wt.) | Specific capacity of the chemical source of current | Specific energy of a chemical source of a current | ||
| A · h / kg | A · h / dm 3 | W · h / kg | W · h / dm 3 | |
| 0 | 57 | 323 | 125 | 710 |
| 1 | 85 | 464 | 197 | 1120 |
| 10 | 65 | 366 | 155 | 878 |
| 20 | 38 | 210 | 91 | 569 |
| thirty | 33 | 185 | 80 | 449 |
Said solid-state current sources with high specific energy characteristics have the following drawbacks:
These sources apply only to primary batteries. In their device described above, the necessary requirements are determined only for the discharge process, when, under the action of the EMF, the fluorine ion is transferred from the cathode by diffusion through the solid phase through the electrolyte to the anode region where the anodic reaction proceeds. This applies only to primary current sources. Cycling of charge / discharge processes, typical for secondary batteries in such a source, is impossible to realize for the following reasons:
1. If after attempting to discharge these current sources try to make a charge, then in the initial charge period, electrolysis of the fluoride of the anode material may occur, with formation of filamentous electron-conducting structures directed to the electrolyte layer in the solid phase. These structures are called dendrites and their formation is determined by the heterogeneity of the ionic conductivity of the anode layer. This is typical for all solid-phase processes. When such dendrites approach the electrolyte, electrolysis of the electrolyte layer begins and when the dendrites of the cathode layer reach the source, the current either breaks down or a very low charge capacity (a few percent of the discharge capacity) and high specific energy characteristics obtained by discharging the primary current source become practically inaccessible .
2. Since these known solid-state current sources with high specific energy characteristics are only primary, the problems of maintaining the mechanical strength of solid-state sources, in particular an anode, a cathode, and an anode / electrolyte interface and cathode / electrolyte in flowing Charge and discharge processes in solid-state current sources. For example, for an anodic reaction of 2La + 6F -6e - > 2LaF 3 , the volume of a solid phase increases by 31% during a discharge, and for a cathodic reaction ![]() Decreases by 37%. For primary known current sources / 1 /, this problem does not occur with a single discharge cycle, especially since known current sources were tested only at high temperature and mechanical stresses relaxed under more favorable conditions. For solid-phase processes in the implementation of charge-discharge cycles, such changes are very critical and can lead, even with several charge-discharge cycles, to the destruction of a solid-state current source.
Decreases by 37%. For primary known current sources / 1 /, this problem does not occur with a single discharge cycle, especially since known current sources were tested only at high temperature and mechanical stresses relaxed under more favorable conditions. For solid-phase processes in the implementation of charge-discharge cycles, such changes are very critical and can lead, even with several charge-discharge cycles, to the destruction of a solid-state current source.
An object of the present invention is to provide a secondary solid-state, safe current source with high specific energy characteristics and a large number of charge-discharge cycles.
The technical result achieved with the use of the claimed secondary solid-state current source is as follows:
- achievement of high specific energy characteristics of secondary batteries up to a level of 500 W · h / kg and 600 W · h / l, ensuring the safe use of such batteries;
- achievement of the number of charge / discharge cycles up to 1000 and
- high safety of electrical energy in the current source due to very low self-discharge at 1-3% per year.
To achieve this goal and the technical result, namely the device of a secondary solid-state current source with high specific energy characteristics, the following device is proposed;
1. The solid-state current source consists of an anode (An 0 ), in the form of a metal or metal alloy, the fluorination of which leads to the formation of fluoride or fluorides with a high isobaric formation potential, an electrolyte in the form of a solid fluorine ion conductor with low electron conductivity and a cathode (KtF 0 ) in the form of a fluoride or a solid solution of fluorides with a low isobaric formation potential with a cathodic reaction at a discharge of KtF 0 + e - → F - + Kt 'and anode in the discharge An 0 + F - → An'F + e - , According to the invention, the anode and the cathode are reversible with respect to fluoride ions with a cathode reaction during charge-discharge: Kt 0 F x + Xe - <---> XF - + Kt 'and anode charge-discharge An 0 + XF - <- -> An'F x + Xe - at voltages below the decomposition voltage of the solid electrolyte, and the anode, electrolyte and cathode contain at least one component that prevents the destruction of the solid-state battery during charge-discharge cycles.
2. To obtain high specific energy characteristics and simultaneously safety in the claimed solid-state current source on the basis of solid fluorine-ion conductors, high-energy current-forming solid-state anode and cathodic reactions are realized.
For this:
The anode in the discharged state of the current source can be made of metals Li, K, Na, Sr, Ba, Ca, Mg, Al, Ce, La or from their alloys, or from their alloys with Pb, Cu, Bi, Cd, Zn, Co, Ni, Cr, Sn, Sb, Fe, and in the charged state of the current source, respectively, from their fluorides.
A solid electrolyte can be made:
- From fluorides La, Ce or from complex fluorides based on them, containing additionally fluoride or fluorides of alkaline earth metals (CaF 2 SrF 2 , BaF 2 ) and (or) alkali metal fluorides (LiF, KF, NaF) and (or) alkali metal chlorides (LiCl, KCl, NaCl),
- Or can be made from complex fluorides based on alkaline earth metal fluorides (CaF 2 , SrF 2 , BaF 2 ), additionally containing rare earth fluorides or (and) alkali metal fluorides (LiF, KF, NaF),
- Or can be based on PbF 2 containing SrF 2 or BaF 2 , or CaF 2 or SnF 2, and a KF additive,
- Or it can be based on BiF 2 containing SrF 2 or BaF 2 , or CaF 2 or SnF 2, and KF additive.
The cathode, which in the charged state of the current source, can be made of simple fluorides: MnF 2 , MnF 3 , TaF 5 , NdF 5 , VF 3 , VF 5 , CuF, CuF 2 , AgF, AgF 2 , BiF 3 , PbF 4 , PbF 4 , CdF 2 , ZnF 2 , CoF 2 , CoF 3 , NiF 2 , CrF 3 , CrF 3 , CrF 5 , GaF 3 , InF 2 , InF 3 , GeF 2 , SnF 2 , SnF 4 , SbF 3 , MoF 5 , WF 5 , fluorinated graphite or from their alloys or from their mixtures, and the discharged state of the current source from Mn, Ta, Nd, VF, Cu, Ag, Bi, Pb, Cd, Zn, Co, Ni, Cr, Ga, In, Ge, Sn, Sb, Mo, W, graphite or from their alloys or from their mixtures.
Table 2 shows the calculated values of the energy parameters of solid-state fluorine-ion current sources with different anode and cathode compositions.
The calculations are based on the following:
For a simplified solid-phase current-forming electrochemical reaction of the type ![]() Flowing in the current source, where
Flowing in the current source, where
Anode: metal - Me
Electrolyte: a solid conductor of fluorine ions with low electronic conductivity;
Cathode: fluoride metal - ![]() ; And the reactions at the electrodes are:
; And the reactions at the electrodes are:
Anode: z · Me + y · F - → Me z F y + y · e -
Cathode: ![]()
E is the voltage of the electrochemical system, or the electromotive force of the electrochemical system (EMF) was calculated from equation (1):
![]()
Where n is the total number of electrons participating in the potential-forming reaction; F is the Faraday number (F = 96485 C / mol); ![]() - change in the Gibbs energy of the reaction, calculated from the Gibbs-Helmholtz equation (2):
- change in the Gibbs energy of the reaction, calculated from the Gibbs-Helmholtz equation (2):
![]()
Where ![]() - a change in the enthalpy and entropy of the chemical reaction at temperature T, respectively
- a change in the enthalpy and entropy of the chemical reaction at temperature T, respectively
W is the specific energy intensity, which is the electric energy at discharge, referred to the unit of mass (W · h / kg) (3):
![]()
Where E is the emf, Cm is the specific electrical capacitance (A · h / kg) calculated from ![]() · Y · F, where
· Y · F, where ![]() - number of moles of active substance (mole), y - number of electrons participating in the anodic reaction, F - number of Faraday (F = 96485 Cl / mol or 26.8 A · h / mole).
- number of moles of active substance (mole), y - number of electrons participating in the anodic reaction, F - number of Faraday (F = 96485 Cl / mol or 26.8 A · h / mole).
W ![]() - the value of the specific volumetric electrical energy (density of electrical energy), which is the electric energy in the discharge, referred to the unit of the current source (W · h / dm 3 ) (4):
- the value of the specific volumetric electrical energy (density of electrical energy), which is the electric energy in the discharge, referred to the unit of the current source (W · h / dm 3 ) (4):
![]()
Where V is the overall volume of the current source, dm 3 .
In Table 2, the parameters of the prior art current source with a lead anode and an AgF cathode are given as a comparison.
From the results given in Table 2, it follows that for the claimed secondary solid-state current source, the proposed anode and cathode compositions can achieve very high specific energy characteristics.
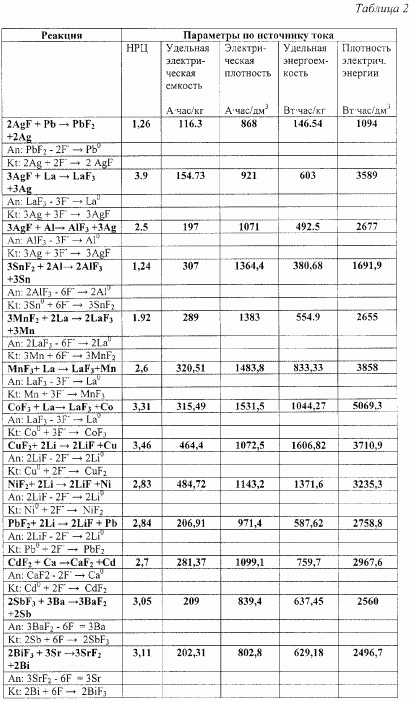 |
3. The solid anode device is reversible with respect to fluorine ions and allows the anodic reversible solid-phase reaction to be realized (in the generalized form: An 0 + XF - <---> An'F x + Xe - ), for which the reduced form of the anode material An 0 has High electronic conductivity, the fluorination of which leads to the formation of An'F x fluoride with a high conductivity of fluoride ions in the solid phase; Or for diffusion of fluorine ions to the anode material (An 0 + XF - ) and the output of electrons into the external circuit of the current source (An'F x + Xe - ), the anode material additionally contains additives providing the necessary for reversible reaction both ionic and electronic Conductivity. 4. The solid cathode device is reversible with respect to fluorine ions and allows the realization of a reversible cathode solid-phase reaction (in the generalized form: Kt 0 F x + Xe - <---> XF - + Kt '), for which the reduced form of the cathode material Kt' has High electronic conductivity, the solid fluorine-containing phase Kt 0 F x has a high conductivity of fluorine ions or to provide diffusion of fluorine ions through the cathode material (XF - + Kt ') and the supply of electrons from the external circuit of the current source (Kt 0 F x + Xe - ) cathodic The material additionally contains additives providing both the ionic and electronic conductivity necessary for the reversible cathodic reaction. 5. The solid electrolyte device makes it possible to realize a high conductivity of fluoride ions in the solid phase at very low or practically absent electron conductivity. The voltage of solid electrolyte decomposition during the charging process should be higher than the voltage of solid-phase electrolysis of the oxidized form of the anode material. This is achieved by optimizing the chemical composition of the solid electrolyte or (and) additional additives to the electrolyte of materials with a low or practically absent electronic conductivity, which increase the decomposition voltage of the electrolyte. 6. The device of the solid-state secondary current source includes an additional component or components that are part of the anode, electrolyte and cathode and prevent destruction of the solid-state battery due to mechanical stresses during charge-discharge cycles. |
Table 3 shows the changes in the volume of the anode and cathode in the charge-discharge cycles of some solid-state current sources from the number stated in the above paragraph 2.
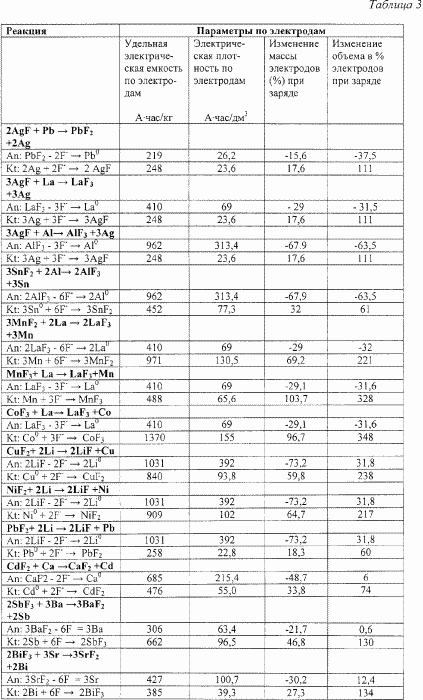 |
The above results show that in the secondary solid-state current source in charge-discharge cycles there are changes in the volume of the anode and cathode, which causes mechanical stresses in the anode, cathode and anode / electrolyte and cathode / electrolyte interfaces. The introduction of an additional component or components will make it possible to strengthen the structure of the current source. This component or components can be made of polymers, for example fluoroplastics, can be made of ionic conductors or (and) glasses. The efficiency of the claimed secondary solid-state current source is as follows: When a current source is discharged, a solid-phase high-energy current-generating reaction is realized with the participation of fluorine ions: when the external circuit is closed at the current-cathode interface, incoming electrons initiate the diffusion of F ions through the internal EMF to the solid phase of the cathode to form the reduced form of the cathode, then after diffusion transfer of fluoride ions By solid electrolyte and their transfer to the anode zone at the anode, a solid-phase interaction of fluoride ions with the anode and the formation of fluoride (oxidized form of the anode) followed by the transfer of electrons to the external circuit. When a current source is charged under the influence of an external electric field on reversible electrodes-the anode and cathode-the following processes occur. Under the influence of an external electric field solid-state electrolysis of the oxidized form of the anode takes place, followed by diffusion of fluoride ions through the electrolyte and fluorination of the reduced phase of the cathode with the transition of electrons to the external circuit. The claimed device of a secondary solid-state current source makes it possible to realize this process and achieve a technical result, namely high specific energy characteristics of secondary batteries with a large number of charge / discharge cycles to a level, ensuring the safety of their use and the long-term preservation of electrical energy. |
USED LITERATURE
1. AA Potanin. "Solid-state chemical current source based on an ionic conductor of the lanthanum fluoride type". Ros. Chem. J. (J. .. D. Chemistry, D. D. Mendeleyev) 2001, vol. 45, No. 5-6, p. 58-63. (prototype).
2. SSPrasad. Deffect structures and anion conducting solid electrolytes. In the book Handbook pp550-552.
3. British Patent No. 1524126, H01M 6/18, 10/36, publ. 06/09/78.
4. The patent of the Russian Federation №2187178 Н 01 М 6/18, 10/36, publ. 10.08.02.
5. The patent of the Russian Federation № 2136083, Н 01 М 6/18, publ. BI № 24, 1999.
6. US Patent No. 6,379,841 B1, H 01 M 4/58, April 30, 2002.
CLAIM
1. A solid secondary current source consisting of an anode in the form of a metal or metal alloy, the fluorination of which leads to the formation of fluoride or fluorides with a high isobaric formation potential, an electrolyte in the form of a solid fluorine ion conductor with high ionic and low electron conductivity and a cathode in A fluoride or a solid solution of fluorides with a low isobaric formation potential, characterized in that the anode and the cathode are reversible with respect to fluoride ions at voltages below the decomposition voltage of the solid electrolyte, and the anode, electrolyte and cathode contain at least one component, Preventing the destruction of solid-state batteries during charge-discharge cycles.
2. The solid-state secondary current source according to claim 1, characterized in that the anode and cathode materials are selected from the condition for performing a reversible cathodic reaction in the charge-discharge mode: KtFx 0 + Xe - <---> XF - + Kt ', and reversible anode Reactions in charge-discharge: An 0 + XF - <---> An'F x + Xe - , where An 0 and KtF x0 - designation of anode material and cathode material in the form of fluoride of a charged current source; An'F x and Kt 'of the corresponding discharged, e - , F - - electron and fluorine ion respectively; X is the number of charge carriers.
3. The solid-state secondary current source according to claim 1, characterized in that the reversibility of the anode and the cathode is ensured by the addition of solid fluorine conductors with high ionic conductivity to their composition.
4. The solid-state secondary current source according to claim 1, characterized in that the reversibility of the anode and the cathode is provided by additional inclusion of solid conductors with high electronic conductivity in their composition.
5. The solid-state secondary current source according to claim 1 or 2, characterized in that the anode in the discharged state of the current source is made of metals Li, K, Na, Sr, Ba, Ca, Mg, Al, Ce, La, or from their alloys , Or from the alloys of these metals with Pb, Cu, Bi, Cd, Zn, Co, Ni, Cr, Sn, Sb, Fe, and in the charged state of the current source, respectively, from their fluorides.
6. The solid-state secondary current source according to claim 1 or 2, characterized in that the cathode in the charged state of the current source is made of fluorides: MnF 2 , MnF 3 , TaF 5 , NdF 5 , VF 3 , VF 5 , CuF, CuF 2 , AgF, AgF2, BiF3, PbF2, PbF4, CdF2, ZnF2, CoF2, CoF3, NiF2, CrF2, CrF3, CrF5, CaF3, InF2, InF3, GeF2, SnF 2 , SnF 4 , SbF 3 , MoF 5 , WF 5 fluorinated graphite, or from their alloys or from their mixtures, and the discharged state of the current source from Mn, Ta, Nd, VF, Cu, Ag, Bi, Pb, Cd , Zn, Co, Ni, Cr, Ga, In, Ge, Sn, Sb, Mo, W, graphite, or from their alloys or from mixtures thereof.
7. The solid-state secondary current source according to claim 1, characterized in that the solid electrolyte is made of La, Ce fluorides or complex fluorides based on them, containing additionally fluoride or fluorides of alkaline earth metals (CaF 2 , SrF 2 , BaF 2 ), and (Or) alkali metal fluorides (LiF, KF, NaF), and (or) alkali metal chlorides (LiCl, KCl, NaCl).
8. The solid-state secondary current source according to claim 1, characterized in that the solid electrolyte is made of complex fluorides based on alkaline earth metal fluorides (CaF 2 , SrF 2 , BaF 2 ), additionally containing rare-earth metal fluorides and / or alkali metal fluorides (LiF, KF, NaF), and (or) alkali metal chlorides (LiCl, KCl, NaCl).
9. The solid-state secondary current source according to claim 1, characterized in that the solid electrolyte is made of fluorides based on PbF 2 containing SrF 2 , or BaF 2 , or CaF 2 , or SnF 2 and KF additive.
10. The solid-state secondary current source according to claim 1, characterized in that the solid electrolyte is made of fluorides based on BiF 3 containing SrF 2 , or BaF 2 , or CaF 2 , or SnF 2 and KF additive.
11. The solid-state secondary current source according to claim 1, characterized in that the solid electrolyte consists of a mixture of two or more solid electrolytes.
12. The solid-state secondary current source according to claim 1, characterized in that polymeric materials that are chemically stable with respect to the electrolyte materials of the anode and cathode in the charge-discharge cycles are used as components preventing the destruction of the solid-state battery during charge-discharge cycles.
13. The solid-state secondary current source according to claim 12, characterized in that fluorine-containing polymers or mixtures thereof are selected as polymer materials chemically resistant to anode and cathode materials in charge-discharge cycles.
14. The solid-state secondary current source according to claim 1, characterized in that solid fluorine conductors are selected as components to prevent the destruction of the solid-state battery during charge-discharge cycles.
15. The solid-state secondary current source according to claim 1, characterized in that a material of the solid fluorine conductor in the form of an electrolyte which is used in the battery is selected as a component preventing destruction of the solid-state battery during charge-discharge cycles.
16. The solid-state secondary current source according to claim 1, characterized in that glass or glass-like materials are selected as components to prevent the destruction of the solid-state battery during charge-discharge cycles.
print version
Date of publication 17.03.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.