| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2187178
![]()
SOLID CHEMICAL CURRENT SOURCE
The name of the inventor: Potanin AA; Vedeneev N.I.
The name of the patent holder: Russian Federal Nuclear Center - All-Russian Scientific Research Institute of Experimental Physics
Address for correspondence: 607190, Nizhny Novgorod Region, Sarov, Mira Avenue, 37, RFNC - VNIIEF, OPINTY, AA Kimachevu
Date of commencement of the patent: 1999.10.05
The invention relates to the field of electrical engineering, in particular to solid-state chemical current sources, and can be used in the production of primary and secondary current sources. SUMMARY OF THE INVENTION: A solid-state chemical current source comprises a lead-based anode, a silver fluoride-based cathode and an electrolyte made of a rare earth fluoride fluoride-containing compound, for example lanthanum trifluoride. In addition, the electrolyte may contain an alkaline earth metal fluoride, for example barium difluoride, and / or an alkali metal fluoride, for example potassium fluoride or lithium fluoride. The invented solid-state chemical current source provides stability of operation in two modes of both primary and secondary current source with an increase in energy intensity 4-8 times in the mode of a secondary current source, has a low self-discharge value, which provides an increase in the time of preservation of electrical energy; There is an increase in the overall characteristics of the current source.
DESCRIPTION OF THE INVENTION
The invention relates to the field of electrical engineering, in particular to solid-state chemical current sources, and can be used both as a primary and secondary source of current.
A solid-state chemical current source [1] is known in which the anode is made of a rare-earth metal metal, for example lanthanum, the cathode is made of ion-conducting complex fluoride, for example bismuth trifluoride with the addition of potassium fluoride and metal oxide. As an electrolyte, a fluorine-containing compound based on rare earth metal fluoride, for example lanthanum trifluoride with barium difluoride, is used.
This chemical current source is operable in the temperature range up to 500 o C. It is characterized by high energy intensity [1].
However, it can only be used as a primary source of current, which significantly reduces the area of practical use of this current source.
The closest to the claimed current source is the known solid-state chemical current source [2], in which the anode is made of lead, the cathode is made of silver fluoride, and the electrolyte is a polycrystalline composition consisting of lead fluoride with the addition of potassium fluoride. The use of an electrode pair of lead-silver fluoride in fluoride batteries is characterized by high reversibility of electrode processes, which allows it to be used both as a primary and secondary source of current. However, this current source, when used in the secondary version, that is, in the battery version, is characterized by low energy intensity. This is due to the fact that when the battery is charged, the electrolysis of anodic lead fluoride and the electrolysis of the solid electrolyte and the lead fluoride, which leads to the destruction of the electrolyte layer, occur. As a consequence, in the secondary source of said device, when carrying out a charge cycle, the realization of a low charging capacity is possible, and as a result, the current source has a low electric capacity. For example, in a known solid-state chemical source of current [2], the electrical capacity is 0.65 mAh. In a similar solid-state current source [3], the electric capacity of the current source is 0.3 mAh. The increase in the electrical capacity of this device can only be achieved by increasing the dimensions, which is not always permissible and justified, since current sources in this case have low specific characteristics.
In addition, to the significant drawbacks of the known current source [2] should be attributed the problem of long-term preservation of electrical energy when used in the battery version. This problem is due to the fact that when the current source is charged, a change in the chemical composition of the electrolyte due to the electrolysis of the electrolyte is possible. In this case, the metal formed in the electrolyte contributes a significant electronic component to the electrical conductivity of the electrolyte. Such a change in turn leads to a self-discharge of the current source, that is, to a decrease in the safety of electrical energy or to the inapplicability of the current source.
The object of the present invention is to increase the energy intensity and increase the time of preservation of electrical energy due to the decrease in the self-discharge of the solid-state current source when it is operated in the battery mode.
The technical result achieved by using the invention is as follows:
- the ability to operate a chemical source of current both in the primary and secondary modes;
- a significant increase in the electrical capacity of the current source when using it in the secondary mode, that is, in the battery mode (4-8 times);
- a decrease in the self-discharge of the current source and, consequently, an increase in the time of preservation of electrical energy.
- increase in the overall characteristics of the current source.
In order to achieve the above object and achieve the above-mentioned technical result in a known solid-state chemical current source comprising a lead-based anode, a cathode based on silver fluoride and an electrolyte made of a fluorine-conducting compound according to the invention as fluorine-conducting compound, it contains at least rare-earth metal fluoride. As a rare earth fluoride, lanthanum trifluoride can be used. In addition, the electrolyte may contain an alkaline earth metal fluoride, for example barium difluoride, and / or an alkali metal fluoride, for example potassium fluoride or lithium fluoride.
The invention corresponds to the criterion of "novelty", since the comparative analysis with the prototype showed that it has distinctive features.
When verifying the technical solution for compliance with the criterion of "inventive level", it is established that RF patent 2136083 [1] is aware of the use in a solid-state current source of electrolyte from a fluorine-containing compound containing rare earth metal fluoride. However, the known device does not provide a technical result, which is achieved in the claimed invention. The known chemical source operates only in the primary mode, and the claimed current source operates in both the primary and secondary current source modes, and high power capacity characteristics are obtained when operating in the secondary current source mode.
On the basis of the analysis, we can conclude that the claimed technical solution meets the criterion of "inventive step".
In the claimed current source, the electrolyte is a solid fluoride conductor based on rare earth fluorides, characterized by high fluorine ion conductivity and low electronic conductivity. The introduction of alkaline earth metal difluorides into fluorides of rare earth metals leads to an increase in ionic conductivity, and the additional introduction of alkali metal fluorides contributes to a further increase in the fluorine conductivity. Using reversible electrodes, a lead-based anode and a cathode based on silver fluoride, and placing between them a solid electrolyte made of a fluorine-containing solid compound based on rare earth fluoride, the device of the claimed current source is realized. This current source can be used in both the primary and secondary current source versions.
When the claimed source is charged, that is, when it is used in the secondary version, only the anode, consisting mainly of lead fluoride, is electrolyzed. A solid electrolyte layer made of a fluorine-containing solid compound based on rare earth metal fluoride does not undergo electrolysis in a wide range of charging voltage.
As a result of this stability of the solid electrolyte, the claimed current source has a higher electrical capacitance compared to the prototype (Table 1). The use of an electrolyte in the current source in the form of a fluorine-conducting solid compound based on rare-earth metal fluoride provides not only the chemical stability of the electrolyte when the current source is charged, but also the preservation of the physical properties of this material. In particular, the low electron conductivity is preserved. Without changing the chemical composition, fluorine-conducting solid compounds based on rare-earth metal fluoride have an electron conductivity of 1 · 10 -8 -1 · 10 -7 Ω -1 · cm -1. This level of electronic conductivity allows to ensure low self-discharge of the claimed current source and, accordingly, high Safety of electrical energy.
Thus, the use of electrolyte in the claimed current source in the form of a fluorine-conducting solid compound based on rare earth metal fluoride makes it possible to improve the characteristics and thereby significantly expand the field of practical use of the current source both in the primary and secondary modes, that is, in the battery mode. At the same time, there is a significant increase in the energy intensity of the current source, a decrease in self-discharge and an increase in the time of preservation of electrical energy when used in the battery mode.
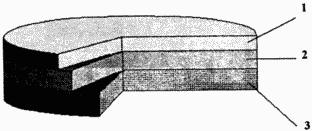 |
The figure shows the scheme of the claimed solid-state current source. It is a three-layer set in contact: |
THE OPERATING PRINCIPLE OF THE PROPOSED SOURCE OF POWER IS IN THE FOLLOWING
In the claimed current source, under the influence of the EMF of the electrode pair, the lead of silver fluoride upon closure of the external circuit takes place the transfer of the fluorine ion through the cathode, then along the solid electrolyte, followed by the anodic interaction of the fluorine ion with lead and the formation of lead fluoride. Due to the high chemical stability, the solid electrolyte used does not undergo changes, which ensures the stability of the discharge characteristics when the claimed current source is used as the primary one.
The claimed current source and is operational in the mode of the secondary current source, that is, the battery. When an external voltage is applied to the current source with a reverse polarity with a value greater than the emf, a steady charge of the current source occurs. In this case, electrolysis of lead fluoride takes place in the anode. The resulting fluorine ion passes through the anode layer, then diffuses through the solid phase of the fluorine-conductive electrolyte containing at least rare-earth metal fluoride, followed by interaction with silver in the cathode to form silver fluoride and transition of the electron to the external charging chain. Since the electrolyte used is significantly superior to the chemical stability of lead fluoride, in particular it has a much higher decomposition potential, during the charging process the electrolyte does not change its chemical composition and retains its electrical properties: high fluorine conductivity and low electronic conductivity. As a consequence, a stable charging process of the claimed current source and its operability in the mode of the secondary current source is realized. At the same time, the stability of the electrolyte in the claimed current source when operating in both primary and secondary mode determines the high safety of electrical energy due to low self-discharge.
To confirm the criterion of "industrial applicability", prototypes of the claimed solid-state current source were made in the form of three-layer elements:
- Anode - based on lead with additives of lead fluoride and potassium fluoride;
- electrolyte - based on lanthanum fluoride with the addition of barium fluoride;
- cathode - based on silver fluoride.
To compare the performance of the claimed current source and the prototype, prototype current source prototypes in the form of three-layer elements were manufactured:
- Anode - based on lead with additives of lead fluoride and potassium fluoride;
- electrolyte - based on lead fluoride and potassium fluoride;
- cathode - based on silver fluoride.
The charge-discharge tests of the current source of the claimed device and the prototype device under the same test conditions and the dimensions of the sources made it possible to compare the electrical capacity of the sources (see table).
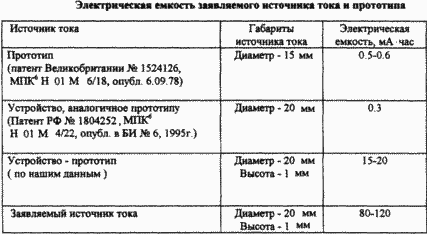 |
As a result of the tests, it was found that the claimed source exceeds the prototype by electric capacity by 4-8 times, while the specific characteristics per unit volume increased significantly; And consequently, the overall characteristics of the current source have improved. In the claimed current source, the solid electrolyte did not change its properties in charge-discharge cycles, providing low self-discharge and safety of electrical energy. In the claimed current source, electrolytes based on trifluorides of other rare earth metals, in particular trifluoride cerium, can be used. The similarity of the chemical properties of solid ionic conductors on their basis allows to solve the task and achieve the indicated technical result by using a fluorine-conducting solid compound based on various fluorides of rare earth metals. |
An important advantage of the claimed current source is that the electrolyte in the form of a fluorine-containing solid compound based on rare earth fluoride is significantly superior in heat resistance to the electrolyte in the form of a fluoride-conducting solid compound based on lead fluoride. If compounds based on rare earth fluoride in the claimed current source are thermally stable to 1500 ° C, the lead fluoride compounds used in the prototype are thermally stable to 400-600 ° C. This circumstance distinguishes the claimed current source as more heat-resistant and safe at Operation.
INFORMATION SOURCES
1. The patent of the Russian Federation 2136083, Н 01 М 6/18, publ. BI, 24, 1999.
2. British Patent 1524126, H01M6 / 18.10 / 36, publ. 09/06/78 (prototype).
3. The patent of the Russian Federation 1804252, Н 01 М 4/22, publ. BI 6, 1995.
CLAIM
A solid chemical current source consisting of a lead-based anode, a cathode containing silver fluoride and an electrolyte made of a fluorine-conducting compound, characterized in that as a fluorine-conducting compound it contains rare earth metal fluoride.
2. The solid-state chemical current source according to claim 1, characterized in that it contains lanthanum trifluoride as the rare earth metal fluoride.
3. The solid-state chemical current source according to claim 1 or 2, characterized in that the electrolyte further comprises at least an alkaline earth metal fluoride.
4. The solid-state chemical current source according to claim 1 or 2, or 3, characterized in that the electrolyte further comprises an alkali metal fluoride.
5. The solid-state chemical current source according to claim 3, characterized in that the electrolyte as an alkaline earth metal fluoride contains barium fluoride.
6. The solid-state chemical current source according to claim 4, characterized in that the electrolyte as alkali metal fluoride contains potassium fluoride or lithium fluoride.
print version
Date of publication 18.03.2007гг




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.