| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2136083
![]()
SOLID chemical current sources
Name of the inventor: Potanin AA .; Vedeneev NI
The name of the patentee: Russian Federal Nuclear Center - All-Russian Research Institute of Experimental Physics - VNIIEF; Potanin Aleksandr Abramovich
Address for correspondence:. 607190, Nizhny Novgorod region, Sarov, 37 Prospect Mira, VNIIEF, Chief OPINTI Kimachevu AA
Starting date of the patent: 1997.07.23
The invention relates to primary batteries autonomous systems for continuous long. According to the invention the current source consists of an anode of a metal from the group of rare earth or an alloy of ftorionprovodyaschego electrolyte consisting of at least one rare earth fluoride and a fluoride of an alkaline earth metal, and a cathode consisting of a mixture of metal oxide and an ion-conductive complex fluoride in the of which, except fluorine includes at least two different metal valency. In the cathode composite fluoride 11,5-96,9 mol.%; metal oxide 3,1-88,5 mol. %. As used oxides of copper oxide (CuO), or lead oxide (PbO 2) or manganese oxide (MnO 2) or vanadium oxide (V 2 O 5), or silver oxide (Ag 2 O). Technical result: the open circuit voltage (OCV) of the current source to 3,9V, operating temperature range from room temperature to 500 ° C.
DESCRIPTION OF THE INVENTION
The invention relates to electrical engineering, in particular, to solid state electrochemical power sources in which an anode, electrolyte, cathode are in solid state.
The inventive current source can be used in the primary battery autonomous for long continuous system at normal temperature; in the primary autonomous power sources for use at high temperatures; composed of various thermal power backup batteries and duration of action, eg for starter run diesel engines.
Known solid state electrochemical power sources (US patent N 4216279, published 05.08.80, H 01 M 6/18, and US patent N 4218527, published 19.08.80, H 01 M 6/18), in which an anode made of lead or its alloys ; used as an electrolyte based on complex fluorides PbF 2 containing SrF 2, or BaF 2, or CaF 2 and KF additive, and PbF 2 and - SnF 2 or PbF 2 - SnF 2 - KF; in the manufacture of a cathode used simple fluorides from the group CoF 3, PbF 3, MnF 3, TaF 3, NdF 5, HgF 2, CuF 2, AgF, AgF 2, BiF 3, in admixture with manganese oxide (US patent N 4216279, H 01 M 6/18) in a molar ratio of MnO 2: metal fluoride of 1: 2.5 and about 3: 1. Manganese oxide used contains water in an amount of from 1 to 25% by weight. U.S. Patent N 4218527, H 01 M 6/18 cathode consists of a mixture of one of the above and the simple fluoride in a molar ratio of lead oxide PbO 2: metal fluoride equal to 3: 1 and 1: 3. The water content of lead oxide from 0.4 to 10% by weight.
The use of current sources in the cathode a mixture of a fluoride and manganese or lead oxide with a water content required for the implementation of the discharge temperature range limits operation of the current source due to the presence of water in the cathode and may cause instability of the power supply characteristics.
A very important disadvantage is that these known current sources are characterized by low power consumption due to the low power consumption of the anode interaction of fluorine with lead. Theoretically, the energy of interaction of the anode lead with fluorine corresponds to 259 A · h / kg or the anode 27,65 F · h / cm 3 anode. These current sources are characterized by a low value of the open circuit voltage (OCV) of 1.36 - 1.94 V, according to the test results reported in U.S. Patent 4,216,279, and 1.85 - 1.95 B, respectively, in U.S. Patent 4,218,527.
The closest to the claimed solid-state current source provides a current source, known from the patent EP 0055135 B1, H 01 M 6/18, wherein the anode metal is selected from Li, Sr, Ba, Ca, Mg, Ce, La or Ce alloys, La , Mg; the solid electrolyte is a composition which includes 70% of lanthanum or cerium mol.triftorida at least one of the fluorides, chlorides or carbonates and alkaline earth metal and one of the fluorides or chlorides of an alkali metal carbonate and the cathode may consist of a complex fluoride ion-conducting, formed by two different valence metals, particularly complex ion-conductive salts such as KBiF 4, TiBiF 4, PbBiF 4.
The disadvantage of this known solution is the low value of the OCV. In particular, the current source with Ce-La anodlom, electrolyte CeF 3 - SrF 2 -LiF, and the cathode 4 on the basis of OCV PbSnF current source is 2.28 V. When used as an anode only lanthanum as a cathode and only PbF 2 OCV value has not changed.
The low value of the known current source RRC impact on reducing its power characteristics, which in turn limits the scope of practical use.
In addition, a significant disadvantage of the known current source should include a limited temperature range of operation it in a solid state. The recovered metal during discharge of the current source in the cathode mass are usually to low melting (Rb, Bi, Tl) and a temperature range of solid-state electrochemical reactions limited by the melting temperature of these metals. Because of the low stability of solid-known current sources have a low resistance to fire, and other emergency situations.
The problem to be solved by the present invention - improving the technical and operating characteristics of the solid-state current source, in particular, improving the current source OCV, power characteristics during its discharge and improving the temperature range of operation of the current source in the solid state.
The technical result achieved when using the claimed invention, the following: the value of LDC increased by 2,74 B (prototype) to 3,9B; power value is increased to two times with respect to the prior art; operating temperature range of the power supply in the solid state is increased from 271 o C (prototype) to 500 o C.
To solve this problem, in a solid-state chemical current source. composed of an anode, electrolyte and cathode ftorionprovodyaschego representing a fluoride ion-conducting complex formed by at least two different metal valencies according to the invention of the cathode metal oxide is additionally introduced at the following component ratio (mol%.)
- Complex fluoride - 11.5 - 96.9%
- Metal Oxide - 3.1 - 88.5%
The inventive current source as the metal oxide in the cathode using copper oxide (CuO), or lead oxide (PbO 2) or manganese oxide (MnO 2) or vanadium oxide (V 2 O 5), or silver oxide (Ag 2 O ). As the ion-conductive composite fluoride cathode may contain a solid bismuth fluoride solution with potassium fluoride or a solid lead fluoride solution with potassium fluoride. The anode is a metal from the group of rare earth metals, or an alloy thereof. The electrolyte is a solid ftorionprovodyaschee substance consisting of at least one rare earth metal fluoride and at least one fluoride of an alkaline earth metal.
The inventive solid-state current-producing reaction current source is realized by transferring fluorine ions from the cathode and anode, their interaction with the material. The possibility of this transfer is determined electromotive force (EMF), which practically corresponds to the experimentally measured value of the open circuit voltage (OCV) of the current source. The flow of current-producing reactions identified fluorinated electrolyte conductivity of the material, the ion conducting composite fluoride and fluoride in the cathode, formed by the reaction of the anode material with fluorine.
It was established experimentally:
1. When using the cathode material of the ion conducting composite fluoride mixture with the oxide there is a significant increase in OCV current source.
2. The use of the solid-state current source cathode as a mixture of complex fluoride ion-conductive metal oxide and results in an increase in discharge voltage, which ultimately determines the increase in specific capacity of the current source discharge.
The source of the analogue [US patents 4,216,279 and 4,218,527], in which, when used as the cathode mixture of simple fluoride and oxides of MnO 2 or PbO 2 must be present in the oxide specific amount of water in the inventive power source, this condition is not necessary . The cathode containing a complex fluoride ion-conducting oxides and metals ftorionnaya conductivity occurs and solid phase is realized claimed stable discharge current source without the presence of water in the oxide. When this oxide during discharge of the current source is reacted with a metal or complex metal fluoride of the cathode, which leads to higher current and power EMF source characteristics.

FIG. 1 shows a current source circuit claimed.
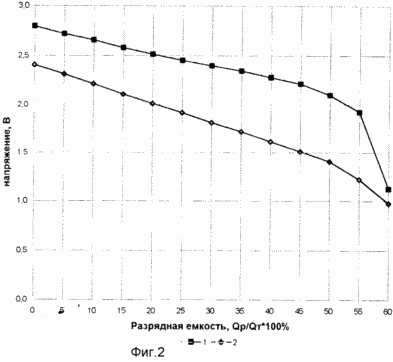
FIG. 2 shows the discharge curve (1) a solid-state current source, wherein the anode used lanthanum, as the electrolyte complex fluoride in the form of a solid solution of LaF 3 - BaF 2 as a cathode ion-conducting complex fluoride in the form of a solid solution of BiF 3 - KF (prototype ), and the discharge curve and (2) said current source, wherein the cathode is used as the ion conducting composite fluoride mixture BiF 3 -KF (90,5% mol.) and CuO (9,5% mol). Discharge current density in both cases was 100 mA / cm 2. The discharge temperature of 500 o C.
Schematically, a current source is in close contact of three layers: 1 - the metallic anode 2 - ftorionoprovodyaschego solid electrolyte 3- solid cathode consisting of a mixture of ion-conductive metal oxide and a complex fluoride, which comprises in addition fluoride, at least two different metal valences.
The operating principle of the proposed current source is as follows. When an external closed-circuit under the effect of the emf fluorine ion complex fluoride of the cathode 3 through the electrolyte 2 ftorionprovodyaschy solid moves to the area of the anode / elekrolit, where the anode metal anode 1 interaction with fluorine to form a substance with a conductivity ftorionnoy. This interaction process is accompanied by the transition of electrons on the outside of a closed circuit. In the process of discharge at the anode is fluorine reacting with the anode material, cathode material is a fluoride complex decomposition with formation F. In this interaction proceeds metal oxide originally introduced into the cathode, with the metals forming the complex fluoride.
Me (a) + m · F - ---> Me (a) F m + m · e - ( anode reaction)
Me '(k) Me' ' (k) F m + m · e - + Me' '' (k) O z ---> Me '(k) Me' '(k) O z + Me' '' (k) + m · F - ( cathodic reaction)
wherein Me (a) - a metal anode;
Me '(k) Me' ' (k) F m - complex fluoride of the cathode;
Me '' '(k) O z - metal oxide cathode material.
Using a current source in the cathode mixture and fluoride ion-conductive complex metal oxides proposed leads to an increase in isobaric isothermal potential-current-producing reactions and increase the EMF current source.
The ionic conductivity of the complex fluoride in the composition of the cathode determines the stability of the discharge current. At the same time there is an increase of the discharge current voltage source. This contributes to the increase of the electronic conductivity of the cathode material due to recovery of the metal of the metal oxide, further introduced into the cathode. Thus, the use in the cathode mixture and fluoride ion-conductive complex metal oxide increases the specific power of the discharge current. When the discharge current source claimed at elevated temperatures in the cathode material occurs, and the oxidation of low-melting metal oxides with the formation of higher melting point, which in turn considerably extends the temperature range of operation of the solid-state current source.
Present a causal relationship between the use of the oxide cathode in a mixture with ionically conductive complex fluoride and increase power supply performance is confirmed by the experimental results.
In the examples, the specific performance of the solid electrolyte is a complex fluoride in the form of a solid solution LaF 3 - BaF 2 containing BaF 2 - 6 mol%. The cathode used in complex fluorides in the form of solid solutions BiF 3 - KF or PbF 2 - KF containing KF - 6 mol%.
All sources of electricity were produced layer by layer by pressing the anode powders, electrolyte and cathode with a force pressing of 8000 kgf / cm 2.
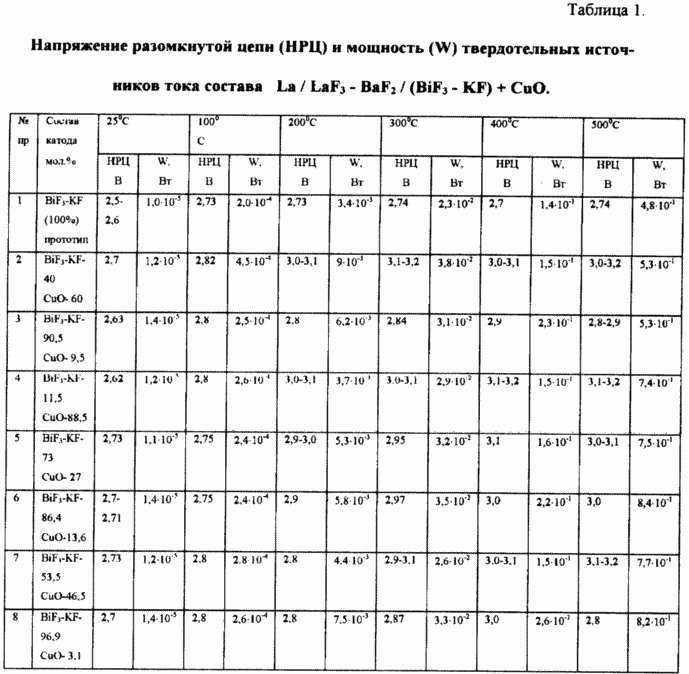
EXAMPLE 1 (Prior Art)
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - BiF 3 - KF
The results of experimental investigations of RRC - 2.6 - 2.8 (T = 25-600 o C)
The results of thermodynamic calculation of EMF - 2.7 - 2.8 (T = 25 - 600 o C).
example 2
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - A mixture BiF 3 - the KF - 40 mol%, CuO -. 60% by mole.
The results of experimental studies OCV - 3.0 - 3.1 V (T = 200-500 o C).
The results of thermodynamic calculation of EMF - 3.1 V (T = 200-500 o C).
example 3
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - A mixture BiF 3 - KF - 90,5% mol, CuO -. 9,5% mol
Tested at the temperature of 500 o C. The value of the density of the electric discharge current was 100 mA / cm 2. 2 shows the discharge curve of the current source solid state (Example 3) and a current source - prototype (Example 1).
EXAMPLES 5 - 8
Solid-state power supply has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - BiF 3 - KF and CuO
Current sources differed molar ratio BiF 3 - KF and CuO, which was 11.5: 88.5; 73:27; 86.4: 13.6; 53.5: 46.5; 96.9: 3.1.
The obtained characteristics of current sources: the OCV and the power (W) in the temperature range of 25 - 500 o C for Examples 1 - 8 are shown in Table. 1 (see Table. 1). The resulting dispersion of OCV values defined manufacturing technology power sources.
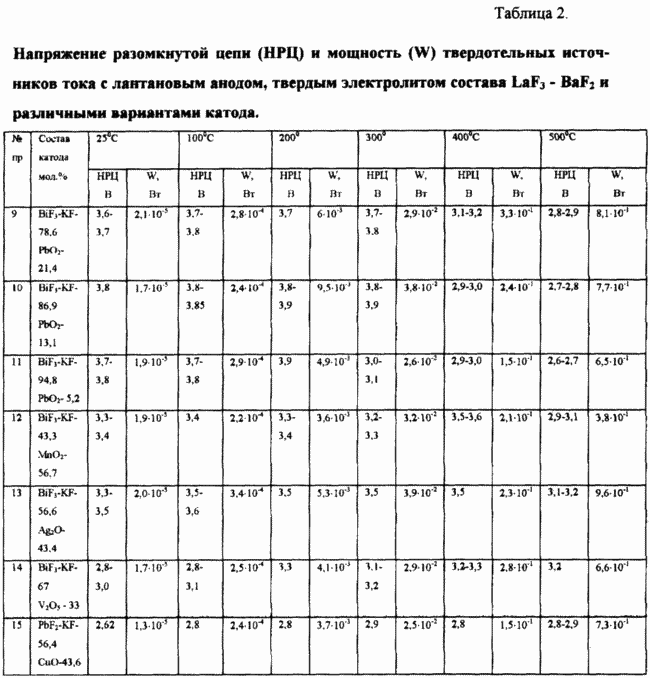
Examples 9 - 11
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - BiF 3 - KF and PbO 2
Current sources differed molar ratio BiF 3 - KF and PbO 2, which was 78.6: 21.4; 86.9: 13.1; 94.8: 5.2.
example 12
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - BiF 3 - the KF - 43.3 mol%, MnO 2 -. 56.7 mole%.
example 13
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - A mixture BiF 3 - KF - 56,6% mol, Al 2 O -. 43,4% mol.
example 14
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - A mixture BiF 3 - the KF - 67 mol%, V 2 O 5 -. 33% by mole.
example 15
The solid-state current source has the composition:
- Anode - La
- Electrolyte - LaF 3 - BaF 2
- Cathode - A mixture of PbF 2 - KF - 56,4% mol, CuO -. 43,6% mol.
The obtained characteristics of current sources: the open circuit voltage (OCV) and the power (W) in the temperature range of 25 - 500 o C of Examples 9 - 15 shown in Table. 2 (see. Table. 2).
Thus, from the examples it follows that the proposed solid-state chemical current source has high performance and is significantly superior to the prototype. The inventive power source is characterized by a high value of OCV. Exceeding the OCV current source claimed by the prototype takes place in a wide temperature range and is 1 - 40%. This increases the power density of the discharge current. This excess in some cases reached up to two times (examples N 9,10).
An important advantage is that the current source claimed by the prototype is superior reliability in the high temperature region T> 271 o C because the cathode reduction products have a higher melting temperature than the solid phase is provided in the cathode during the discharge stability.
In the claimed range oxides introduced into the cathode, highlight PbO 2, MnO 2 and Ag 2 O, which allows the use of more current source OCV increase. At the same time, we should highlight the use of the advantage of the cathode oxide CuO, V 2 O 5 and Ag 2 O, allowing to have a consistently high OCV up to 500 o C. This characteristic is very important, as this not only expands the temperature range of operation of the current source but also extend technological capabilities of the high temperature heat treatment of the current sources to reduce their internal electrical resistance.
CLAIM
1. Solid-state chemical current source consisting of a solid anode, cathode and electrolyte ftorionprovodyaschego a fluoride ion-conductive compound formed by at least two different valence metals, characterized in that the composition additionally introduced cathode metal oxide in the following ratio, mol.% :
- Complex fluoride - 11.5 - 96.9
- Metal Oxide - 3.1 - 88.5
2. The solid-state chemical current source according to claim 1, characterized in that the cathode comprises a metal oxide as copper oxide or lead oxide or manganese oxide, or vanadium oxide or silver oxide.
3. The solid-state chemical current source according to claim 1 or 2, characterized in that the solid electrolyte consists of at least one rare earth metal fluoride, and at least one fluoride of an alkaline earth metal.
4. The solid-state chemical current source according to claim 1, 2 or 3, characterized in that the anode material is selected from the group of rare earth metals or their alloys.
5. The solid-state chemical current source according to claim 1, characterized in that the cathode comprises a complex fluoride in the form of a solid solution of bismuth fluoride and potassium fluoride.
6. The solid-state chemical current source according to claim 1, characterized in that the cathode comprises a complex fluoride in the form of a solid solution of lead fluoride and potassium fluoride.
print version
Publication date 06.02.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.