| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2119964
![]()
METHOD OF EXTRACTING NOBLE METALS AND INSTALLATION
FOR ITS IMPLEMENTATION
The name of the inventor: Petrova EA; Samakhov AA; Makarenko M.G.
The name of the patent holder: Open Joint-Stock Company "Catalyst"
Address for correspondence:
Date of commencement of the patent: 1997.12.05
The invention relates to methods for recovering precious metals from spent catalysts, and to electrochemical processes with a fluidized or fixed bed.
The method for extracting precious metals from spent catalysts, slurries, concentrates and other materials with an inorganic base provides for leaching in the electrolyte , deposition of metals in an electrochemical cell with a backfilling cathode and subsequent liberation of the noble metal from the cathode by known methods. Leaching of precious metals and their deposition on the backfill cathode is carried out simultaneously in a single stage when the electrolyte is circulated through a stationary filter or suspended layer of leached material particles and a cathode filled electrolyzer, which makes it easier to operate the unit. The plant for extracting precious metals from spent catalysts, slurries, concentrates and other materials with an inorganic base contains a leaching unit, an electrolytic cell for depositing metal with a cathode, an insoluble anode, a control valve and a stop valve. The leaching unit is connected to an electrolyzer for depositing a metal with a cathodic cathode filled with pipelines with means for circulating the electrolyte between them, the leaching unit consisting of one or more reactors and the charging cathode of the cell connected to the device for its overload.
DESCRIPTION OF THE INVENTION
The invention relates to methods for recovering precious metals from spent catalysts, and to electrochemical processes with a fluidized or fixed bed.
Noble metals of the platinum group, especially platinum, palladium, silver and rhodium are widely used as catalysts in refining and petrochemistry, in the nitrogen industry, for neutralizing industrial waste gases and car exhaust gases, internal combustion engines, in fuel electrochemical cells and . Because of the limited resources and high cost of precious metals, their recovery from catalysts that have worked out their time is an important task. In industrial catalysts, in most cases noble metals or mixtures thereof are applied to solid porous supports with highly developed surface - aluminum oxides, silicon, aluminosilicates, carbon, etc. The noble metal content of such catalysts is 0.1-5.0 % by weight . Spent catalysts often contain contaminants in the form of polymer-coke deposits formed as a result of side reactions and / or in the form of foreign impurities coming from the processed raw materials or corrosion products of the equipment.
The above circumstances significantly complicate the technology of recovery of precious metals from spent catalysts and do not allow the use of known gravity and flotation methods for concentrating noble metals from ore materials. Recuperation of precious metals from spent catalysts is currently carried out mainly pyrometallurgical or hydrometallurgical methods.
Hydrometallurgical methods are mainly implemented in the following variants.
A method is known for processing objects containing noble metals [1], which involves the processing of the object to obtain noble metals in a sorbed form and sorption by a polymeric nitrogen-containing organic sorbent in an electrochemical apparatus. The sorbent and solution are fed into the interelectrode space of the electrochemical apparatus.
The disadvantage of the method is the complexity of its implementation, an insufficiently high degree of extraction of precious metals. In addition, the sorbent is an expensive product.
An electrolyzer is known for extracting metals from solutions of their salts containing alternating anodic and cathodic blocks with an aperture in the cathode block for introducing the initial solution. The cathode blocks are made in the form of chambers with perforated walls serving as a current lead, on which cathodes of carbon graphite material are fixed.
The disadvantage of the cell is the insufficiently high recovery of precious metals [2].
The closest solution is the method [3], in which a method for the regeneration of noble metals - platinum, palladium, rhodium or their mixtures - from spent catalysts and an installation for its realization are proposed [3].
This method consists in preliminarily leaching precious metals from particles of crushed material in the anode compartment of an electrolyser with an anion exchange membrane, with catalyst carriers or substrates being non-conductive materials. As the electrolyte, hydrochloric acid with a concentration of 5-35% is used, and the leaching is carried out in a stationary filtering layer of the material particles or in the fluidized bed during circulation of the electrolyte through a bed of leachate material. In the second stage, the noble metals from the leach solution are precipitated on carbon particles in a fluidized state in the cathode compartment of a second electrolysis cell with a cation exchange membrane. Finally, in the third stage, the precious metals are again leached from the resulting material by anodic dissolution in the fluidized bed. In this case, sufficiently concentrated solutions for noble metals are obtained, which can be used to prepare the corresponding catalysts. In methods based on leaching, the consumption of reagents is much less than in dissolution, but to completely recover noble metals from porous carrier particles, multiple washing of the precipitate is necessary.
The disadvantage of the process is the low concentration of precious metals in leach solutions, which makes it difficult to separate metals from solutions and increases losses. The installation used to implement the energy-intensive method has a large amount of sewage.
It is an object of the present invention to provide an efficient method for recovering precious metals from spent catalysts using leaching and to provide an easy-to-use plant for implementing this method.
The problem is solved by a method for extracting precious metals from spent catalysts, sludges, concentrates and other materials with an inorganic base including leaching in the electrolyte, deposition of metals in an electrolytic cell with a backfilling cathode, and subsequent liberation of the noble metal from the cathode by known methods. The leaching of noble metals and deposition of them on the cathode are carried out simultaneously in one stage during circulation of the electrolyte through a stationary filter or suspended layer of particles of the leachate and an electrolytic cell with a cathodic charge.
The electrolyte is an aqueous solution of sodium chloride with a concentration of 10-25% by weight, containing hydrochloric acid or alkali.
The filler cathode contains activated carbon.
The noble metal is isolated by ashing the cathode material or by anodic dissolution of the metal.
The leachate contains platinum, palladium, silver, rhodium, or mixtures thereof.
The plant for extracting precious metals from spent catalysts, slurries, concentrates and other materials with an inorganic base contains a leaching unit, an electrolytic cell for depositing metal with a cathode, an insoluble anode, a control valve and a stop valve. The leaching unit is connected to an electrolyzer for depositing a metal with a cathodic cathode filled with pipelines with means for circulating the electrolyte between them, the leaching unit consisting of one or more reactors and the charging cathode of the cell connected to the device for its overload.
The reactor for leaching in the stationary filter bed of the particles of the leachate is equipped with a flow distributor.
The leaching reactor is provided with means for maintaining the material in a suspended state.
The leaching unit contains a pH measuring chamber, an electrolyte tank with a flow regulator.
The installation contains an automatic control system.
The installation comprises a device for loading and discharging the leachate into the leach apparatus.
As shown by our studies, when leaching precious metals from particles with a base of non-conductive substances, the leaching of the material in the anode chamber in the electric field of the cell or outside it slightly affects the rate and depth of the leaching. At the same time, placing the leachate in the electrolyzer between the anode and the cathode substantially increases the electrical resistance of the cell and, accordingly, increases the power consumption. It has also been found that the preferred cathode material is activated carbon. Compared to carbon-graphite fibrous materials, it makes it possible to achieve lower residual concentrations of noble metals in the catholyte, apparently with the sorption properties of activated carbon.
As an electrolyte, in the present invention, unlike the prototype, aqueous solutions of sodium chloride with a concentration of 10-25% are used and containing hydrochloric acid or alkali in an amount necessary to conduct the process.
The proposed set of features of the method of extraction of noble metals and an installation for its implementation leads to the achievement of the task.
The extraction of precious metals by the proposed method occurs when the electrolyte is circulated through a leaching unit and an electrolytic cell with a cathodic charge at the same time, while noble metals are accumulated on the cathode. Then the charging cathode is discharged from the cell for further processing, for example, the ashing of the cathode material is made, the metal is extracted or without unloading the cathode from the cell. In the latter case, the noble metal is dissolved by passing an electric current of reverse polarity and sufficiently concentrated salt solutions are obtained.
In the drawing, an installation diagram for implementing the proposed method is shown.
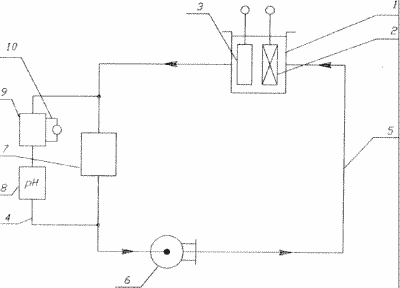
The apparatus comprises an electrolytic cell 1 with a cathode filling 2 and an insoluble anode 3. The electrolyzer 1 is provided with any known device for loading and unloading the cathode 2 (not shown in the diagram). The leaching unit 4 is connected by a line of conduits 5 with an electrolyzer 1 for circulating the electrolyte between them by using known means, for example pump 6.
The leaching assembly comprises one or more reactors 7 which are provided with any known devices for loading and discharging the leachate (not shown in the diagram).
The leaching unit is provided with a pH measuring chamber 8, an electrolyte tank 9 with an automatic flow control regulator 10. The leaching reactor 7 in the stationary filter bed of the leachate particles is provided with a flow distributor, for example a blade impeller. The reactor 7, when working with a material in a suspended state, is provided with known means used for this purpose.
INSTALLATION WORKS AS FOLLOWING
A certain portion of the material is loaded into the reactor 7, through which the electrolyte circulates through the pipelines 5 via a pump 6 through an electrolytic cell 1 filled with cathode 2 filled with activated charcoal granules.
The particulate material in the reactor 7 is continuously suspended by leaching in the suspended bed. The process can also be carried out with a stationary filtering bed of catalyst particles. The required flow rate of the electrolyte from the tank 9 is maintained through a flow controller 10. The set process mode can be maintained using known automated control systems.
After the accumulation on the filling cathode 2 of a sufficient amount of recoverable metal, the cathode 2 by means of a special device, which can be used as any known device for transferring the raw material to the reactor, is overloaded.
At anodic dissolution of the metal, the process is stopped, the electrolyte is drained from the cell 1, washed with a cathode filled with warm water, the cell is filled with a solution of HCl or HNO 3, and the polarity of the electric current is reversed. When the polarity of the current changes, the metal accumulated on the cathode is gradually dissolved.
The following are specific examples of the implementation of the proposed method for recovering precious metals.
Example 1
Palladium recovery from spent catalyst in a stationary filter bed (see drawing).
100 g of the spent alumopalladium catalyst containing 1.8% palladium, after preliminary preparation, in the form of particles of 0.8-2.0 mm in size, is placed in a height of 15 cm in a leaching reactor 7 connected to an electrochemical cell 1 with a cathode filled with a cathode 4 Cm, filled with activated charcoal granules.
The electrolyte is a 15% aqueous solution of sodium chloride, acidified with hydrochloric acid, which by means of pump 6 is constantly circulated between the cell and the reactor with the leachate. After 11-12 hours, the solution leaving the reactor had a residual palladium concentration in the electrolyte of less than 1 mg / dm 3 , the degree of extraction of palladium from the spent catalyst by the analysis of the residue of 99.6-98.9% .
After several experiments in the above mode, without changing the electrolyte and the cathode, the activated carbon with the precipitated metal is removed from the cell, washed with water from the electrolyte residues, dried at 120 ° C., and ash-treated in a muffle furnace at 600-650 ° C. A concentrate with a palladium content of 96-97% is obtained.
Example 2
Palladium recuperation from spent alumopalladium catalyst in suspension.
100 grams of spent alumopalladium catalyst containing 1.78% palladium, after preliminary preparation, in the form of a powder with a particle size of less than 0.5 mm , was suspended in a reactor with mechanical stirring in 600 cc of anolyte coming from the cell as in Example 1 .
The suspension in the reactor is stirred for 1.5 hours , after which the palladium-containing solution is fed to the flow cell, the palladium is deposited on the cathode, and the anolyte is fed to re-leach the palladium from the same batch of material. The degree of extraction of palladium was 99.6% . At the end of the palladium leaching, the remaining carrier is discharged from the apparatus and the process continues with the next portion of the noble metal-containing material as described above, without replacing the electrolyte and activated carbon in the cathode compartment of the cell. After leaching of palladium from several portions of raw material, the contents of the cathode filler are discharged, washed with water, dried and aseptified as in Example 1 . A concentrate containing 94.5% palladium is obtained.
Example 3
Recovery of platinum from the spent catalyst.
50 g of spent catalyst for afterburning of organic substances in production exhaust gases containing 0.42% of platinum on a ceramic honeycomb carrier in the form of a powder with a particle size of less than 0.5 mm is leached in suspension at a ratio of T: H = 1: 3.5 ( By weight) in a reactor equipped with a mechanical stirrer and connected to a flowing electrolyzer as indicated in Example 1 . Electrolyte - an acidic solution of sodium chloride - continuously circulates in the system between the reactor and the electrolyzer. The total volume of electrolyte in the system is 140 cm 3 .
The process continues for 9 hours to a negative qualitative reaction to the presence of platinum in the electrolyte. Extent of extraction of platinum by residue analysis 98% .
Example 4
Recovery of precious metals from an aluminoplatinoride catalyst.
A spent aluminoplatinorodic catalyst for the exhaust gas cleaning of internal combustion engines (pilot batch) containing 0.10% of rhodium and 0.45% of platinum on alumina, in the form of a powder with particle sizes of less than 0.5 mm , is treated in portions of 50 g as Is described in Example 3 .
After 10 hours, the extraction rate of platinum is 95-96% , rhodium 80-85% (based on the analysis of the residue). The total content of platinum and rhodium in the concentrate after ashing is 95% by weight .
Example 5
Extraction of silver from the spent catalyst in a stationary filtering bed.
200 g of spent oxide catalyst containing 4.3% of silver and iron, cobalt, aluminum and manganese oxides, after preliminary preparation, in the form of particles of 0.8-2.0 mm in size, are treated in the same manner as in Example 1 , but with an electrolyte Is a 25% solution of sodium chloride with the addition of sodium hydroxide to pH 8-10 .
After 40 hours, the silver recovery rate is 95-97% , with the remaining components of the catalyst being practically insoluble.
Example 6 (prototype)
In the process for recovering precious metals, leaching is carried out separately in the anode compartment of an electrolyser with an anion exchange membrane. Hydrochloric acid with a concentration of 15% is used as the electrolyte.
Then, the solution obtained after leaching is sent to the second stage into the cathode compartment of a second electrolysis cell with a cation exchange membrane. Then, a third step is carried out and the platinum is recovered by an anodic dissolution leaching.
The concentration of platinum was 226 mg / l , the recovery rate of 98% .
As can be seen from the examples given, the proposed method of extraction of precious metals and the installation for its implementation allow to extract them with a high degree, the amount of sewage considerably decreases, the installation is easy to operate, it allows to organize the process in a continuous mode.
LITERATURE
Patent of the Russian Federation No. 2042719, cl. C 22 B 3/24, 1995.
Author's certificate of the USSR N 395497, cl. C 25 C 7/00, 1973.
U.S. Patent No. 4,775,452, Cl. C 25 F 5/00, 1988.
CLAIM
A method for recovering precious metals from spent catalysts, slurries, concentrates and other materials with an inorganic base, including leaching in an electrolyte, depositing metals in an electrochemical cell with a backfilling cathode, and then separating the noble metal from the cathode by known methods, characterized in that leaching of precious metals and precipitation of them On the backfill cathode is carried out simultaneously in one stage during circulation of the electrolyte through a stationary filter or suspended layer of particles of the leachable material and an electrolytic cell with a cathode filled.
The process according to claim 1, characterized in that an aqueous solution of sodium chloride with a concentration of 10-25% by weight containing hydrochloric acid or alkali is used as the electrolyte.
A method according to claim 1, characterized in that the charging cathode contains activated carbon.
The method according to claim 1, characterized in that the noble metal is recovered by ashing the cathode material or anodically dissolving the metal.
The method of claim 1, wherein the leachate contains platinum, palladium, silver, rhodium, or mixtures thereof.
A unit for extracting precious metals from spent catalysts, slurries, concentrates and other materials with an inorganic base, comprising a leach assembly, an electrolytic cell for depositing metal with a cathode, an insoluble anode, a control and shut-off valve, characterized in that the leaching unit is connected to an electrolyzer for Depositing a metal with a cathodic cathode filled with a line of pipelines with means for circulating the electrolyte between them, the leaching unit consisting of one or more reactors, and the charge cathode of the cell connected to the device for its overload.
A plant according to claim 6, characterized in that the leach reactor in the stationary filter bed of the leachate particles is provided with a flow distributor.
A plant according to claim 6, characterized in that the leach reactor is provided with means for maintaining the material in a suspended state.
The plant of claim 6, wherein the leaching unit comprises a pH measuring chamber, an electrolyte tank with a flow regulator.
The plant of claim 6, characterized in that it comprises an automatic control system.
A plant according to claim 6, characterized in that it comprises a device for loading and discharging the leachate into a leaching apparatus and reloading the cathode from the cell.
print version
Date of publication 07.11.2006гг




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.