| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2109088
![]()
MULTI-BLOCK STEEL ELECTROLYSER FOR EXTRACTION OF METALS
FROM SOLUTIONS OF THEIR SALTS
The name of the inventor: Korenevsky Alexander Dmitrievich; Dmitriev Vladimir Alexandrovich; Kryachko
The name of the patent holder: Korenevsky Alexander Dmitrievich; Dmitriev Vladimir Alexandrovich; Kryachko Konstantin Nikolaevich; Mamilov Vladimir Viktorovich
Address for correspondence:
Date of commencement of the patent: 1996.07.11
Use: The invention relates to hydrometallurgy, namely to the technique of extracting metals from various process solutions by electrolytic method, and can be used to neutralize toxic components of the solution.
Essence: the cathode blocks of the electrolyzer are made in the form of a chamber with grid currents fixed on it on both sides, representing a metal frame with geometric dimensions that coincide with the dimensions of the chamber and divided into sectors by vertical or horizontal bridges.
DESCRIPTION OF THE INVENTION
The inventions relate to hydrometallurgy, namely to the technique of extracting metals from various technological solutions by electrolytic method, and can be used to neutralize toxic components of the solution.
A multi-unit flow-through electrolyzer is known , comprising a housing with anodic and cathodic blocks housed in it, located at the top of the housing by pipelines for supplying solutions and pockets for their removal [1], and a multi-unit flow cell, differing from the first in that, in order to service and provide an opportunity Automation, pipelines for the supply of solutions are located one in the other and located under the bottom of the housing [2].
In known electrolytic cells, the cathode blocks are made in the form of a chamber of a rectangular frame, on both sides of which there are cathodes of several layers of fibrous carbon graphite material laid on perforated metal conductors. To enter the treated solution, the cathode block is provided with a nozzle located at the top [1] or the bottom [2] of the chamber.
The metal-containing solution is fed through the nipple into the space between the two cathodes, passes through the perforated conductors, cathodes from the carbon graphite material, and, depleted during the electrolysis, is removed from the cathode chamber.
However, conventional electrolytic cells are not sufficiently effective, because of the way for supplying current to the cathode through the perforated conductor disposed on the feed side of the treated solution (the back in relation to the counter electrode), which reduces the deposition of metal in the cathode deposit by substantially shielding the back of the cathode side of the treated solution, neravnodostupnosti cathode surface, the unevenness of the hydrodynamic conditions of the cathode by the electrolysis cathode depth.
These shortcomings lead to the advantageous use of known electrolyzers in the autonomous systems from leaching and the technological schemes closed by the processed solutions (repeated circulation): leaching-sorption of valuable components by ion exchange resin or activated carbon-desorption of valuable components by thiourea-electrolysis of rich, for example, Au solutions Up to 2 g per liter) and significantly limit their application in open-type technological schemes: leaching - electrolysis of productive solutions - until the "blank" solution is strengthened with leaching reagents.
The purpose of the invention is to intensify the process of electrolytic extraction of metals from solutions of their salts and to expand the field of application of the electrolytic method of extracting metals into industry.
These results are achieved by the fact that the cathode blocks of the cell are made in the form of a chamber with lattice current conductors fixed on it on both sides, representing a metal frame with geometric dimensions coinciding with the dimensions of the chamber and divided into sectors by vertical or horizontal metal bridges ( Figure 1 ) .
The parameter lattice current lead L (fig. 1), equal to the size of the smallest geometric sectors bus system, determined by the characteristics used as a carbon and graphite cathodes fibrous material so as to ohmic resistance of the cathode bases did not prevent the polarization of the cathode over the entire volume.
The use of a grid current conductor in the cell design, compared with a perforated conductor over the area of the cathode from the supply side of the solution in known electrolytic cells, has the following advantages: the working area of the cathode increases by an order of magnitude, the cathode surface of the cathode shielded to a much lesser degree The intensity of the process of electrolytic deposition of metals over the entire thickness and cathode area, the surface of the cathode is more equally accessible, uniform hydrodynamic conditions of the process along the depth of the cathode are provided, which under comparable conditions causes a higher extraction of metals from the treated solutions than in the known electrolysers Not less than 99.6% of precious metals).
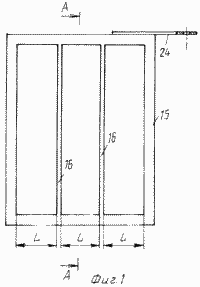 FIG. 1 shows a proposed grid current conductor |
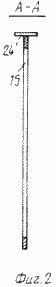 FIG. 2 is a sectional view of A-A of FIG. 1 |
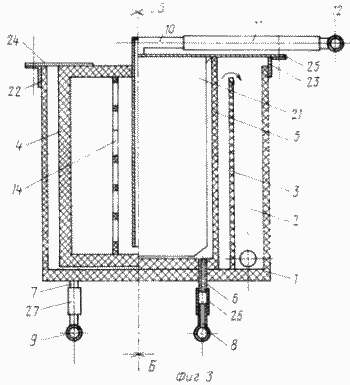 FIG. 3 - cell general view |
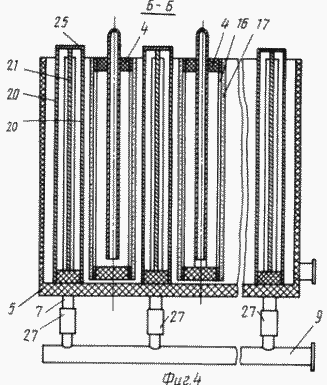 FIG. 4 is a cross-sectional view of the BB in FIG. 3 |
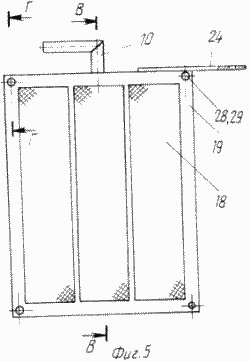 FIG. 5 - cathode block assembly |
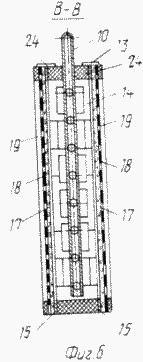 FIG. 6 is a cross-sectional view taken along line B-B of FIG. 5 |
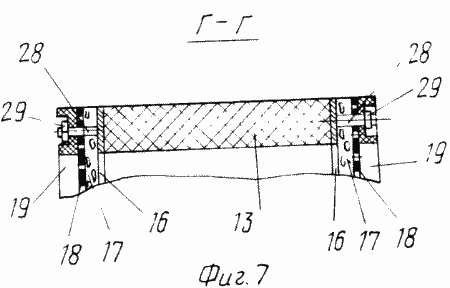
FIG. 7 is a section of FIG. 5.
The cell contains a body 1 with a pocket 2, the inner wall 3 of the pocket 2 being lower than the walls of the body 1 ( Figure 3 ). In the housing 1, cathode and anode blocks 4 and 5 are alternately arranged ( Figures 3, 4 ). The anode blocks 5 are connected to the supply line 8 by means of the fittings 6, by means of the fittings 7 - to the discharge conduit 9. The cathode blocks 4 are connected to the supply pipe of the solution 12 to be treated by means of the nozzle 10 ( FIG .
The cathode blocks 4 are made in the form of chambers which are a frame 13 ( Figure 6 ) made of a non-conductive material, for example a viniplast with perforated baffles 14 of the same material, corresponding to the arrangement of the bridges 16 of the grid conductors 15 ( Figure 1 ) fixed to both sides Framework 13.
The cathodes 17 of fibrous carbon-graphite material, pressed by a mesh 18 of non-conductive material (viniplast) fixed with lattice lids 19 with holes for studs 28 (Figures 6, 7), are fixed on the conductors 15 provided with pins 28 (Fig. The tightness of the cathode block 4 is ensured by tightening the bag with the pins 28 and nuts 29 (Figure 7).
The anode blocks 5 are made in the form of chambers with walls of ion-exchange membranes 20, inside which the anodes 21 ( Fig. 4 ) are located. The chambers are provided with fittings 6 and 7 for the supply and removal of the anolyte, respectively, the anolyte outlet 7 is elongated from the inside and determines the anolyte level in the anode block 5 ( Figures 3, 4 ).
The current lead to the electrode block is carried out via the bus bars 22 and 23 by the petals 24 and 25 ( Fig.3 ).
ELECTROLYSER WORKS AS FOLLOWING
Through the inlet fitting 8 and the conductor 6 is supplied to the anode blocks anolyte solution, which bathes the anode 21 and through the sleeve 7 and the discharge pipe 9 out of the cell.
The metal-containing solution-catholide through the supply line 12 and the nipple 10 is fed into the cathode block 4, passes through the grid current conductors 15, the cathodes 17 and, depleted during the electrolysis, enters the housing 1, from which it pours through the inner wall 3 and enters the pocket 2.
To replace the cathode block 4 shut off the solution, the petals 24 are disconnected from the bus 22 and are replacing cathode block, continuous cathodic electrolysis in the remaining blocks.
The use of the present invention makes it possible to electrolytically extract metals from solutions of their salts continuously over a treatment solution with a different content of valuable components, for example, gold from 2 to 3 mg / l to 2 g / l and to obtain reject (recycled) solutions of the recoverable metals, for example On gold no more than 0,1 - 0,2 mg / l for one cycle, that considerably expands the field of application of electrolytic method of metal extraction in the industry. Furthermore, the use of the proposed technical solution allows to reduce both capital and operating costs in the recovery of metals from solutions.
INFORMATION SOURCES
Author's certificate of the USSR N 395497, cl. C 25 C 7/00, 1972.
Author's certificate of the USSR N 648658, cl. C 25 C 7/00, 1977.
CLAIM
A multi-unit flow cell for extracting metals from solutions of their salts, comprising a housing with anodic and cathodic blocks housed therein, pipelines for supplying and removing anolyte, catholyte supply and a pocket for its withdrawal, characterized in that the cathode blocks are in the form of chambers with lattice current leads , Which is a metal frame with vertical and / or horizontal bridges on current leads of cathodes made of fibrous carbon-graphite material.
print version
Date of publication 07.11.2006гг




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.