| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2255128
![]()
METHOD OF EXTRACTION OF PALLADIUM FROM WASTE
Name of the inventor: Demin Yu.V. (RU); Melnikov SI (RU); Sysoev YM (RU); Kvaratskhelia JK (RU)
The name of the patentee: "Company" Oriya "(RU)
Address for correspondence: 115409, Moscow, p / 38
Starting date of the patent: 2003.08.04
The invention relates to a hydrometallurgical precious metal, in particular methods for direct extraction of palladium from waste electronic, chemical, electrochemical and jewelery industry. The method comprises palladium dissolution and reconstitution of its solutions. Dissolution are aqueous solution containing 100-140 g / l potassium iodide, 60-80 g / l iodine, 20-40 g / l of triethanolamine and 5-20 g / l of potassium hydroxide, and the process is carried out at pH = 11.7 . Process temperature 20-40 ° C. The technical result is to increase the selectivity of the dissolution of palladium.
DESCRIPTION OF THE INVENTION
The invention relates to a hydrometallurgical precious metal, in particular methods for direct extraction of palladium from waste electronic, chemical, electrochemical and jewelery industry.
Known methods for palladium dissolution in concentrated sulfuric acid, nitric acid, mixtures of nitric and hydrochloric acids [1, 2]. Minor amounts of palladium (less than 5.10%) pass into solution in the iodine-iodide medium [3]. To separate the palladium from the solution obtained is added thereto, or various reducing agents (organic and inorganic), or substances that give insoluble palladium compound. Palladium complete dissolution process is generally carried out at elevated temperature (70-90 ° C) for a long time (2-3 hours, and sometimes much more).
The disadvantages of these methods are: low selectivity of the processes (together with palladium in solution substantially all the elements associated to it), which greatly complicates, and thus makes it more expensive process of extracting palladium from the solution; high corrosion aggressiveness of solutions; toxicity of solutions and vapors. It is known that electronic scrap coated with precious metals has basically beryllium bronze containing from 0.7% to 2.5% beryllium. Nearly all the known processes beryllium dissolution of palladium into the solution together with the palladium, which largely determines the toxicity produced technological solutions and vapors.
The closest to the proposed technical essence and achieved result is a process for extracting palladium from the sludge of electrolytic production, based on dissolution of the sludge 3-4 mol / l of nitric acid in the presence of oxalic acid at a temperature of 80-90 ° C, and followed by reduction of palladium from the solution zinc or hydrogen [4].
Disadvantages of the prototype method is dissolving the low selectivity, high corrosiveness processing solutions and processing solutions and toxicity and vapor.
The technical result of the invention is to improve the selectivity of palladium dissolution, reduced corrosiveness and toxicity of the technological solutions and vapors.
Technical result is achieved by a method for palladium recovery from wastes, comprising dissolving palladium and reduction, are dissolved an aqueous solution containing 100-140 g / l potassium iodide, 60-80 g / l iodine, 20-40 g / l of triethanolamine and 5-20 g / l of potassium hydroxide. The process is conducted at pH 7-11 and a temperature of 20-40 ° C.
The main difference of this invention is that the proposed method for the direct dissolution of palladium from the surface of electronic waste, the chemical, electrochemical and jewelery industry, an aqueous solution of potassium iodide, iodine, and triethanolamine.
The method allows for palladium dissolution in an alkaline medium at room temperature. When this process is more selective (in solution hardly pass non-ferrous metals), and the toxicity and corrosiveness technological solutions and vapor are significantly reduced, which is important in the operation of the proposed method.
Using the method for the extraction of palladium for 20 minutes reaches 90%, the copper goes into solution only in the amount of 2% -3%, tin - 2% beryllium - is practically in solution does not pass.
At the same time significantly reduced corrosiveness and toxicity of technological solutions and vapors. In addition (the processing catalysts based on aluminum oxide), is formed are difficult to precipitate.
According to the invention the method is performed as follows. Weigh other electronic scrap or recycled treated with an aqueous solution of potassium iodide, iodine, potassium hydroxide, triethanolamine and the indicated concentration at room temperature for 15-20 min. The filtered solution was mixed with productive to restore dissolved hydrazine iodide and a palladium metal to palladium metal deposition from solution.
USE EXAMPLE
100 g of electronic scrap, comprising 1.5-1.7% of palladium supported on the basis of beryllium copper alloy, treated with an aqueous solution of potassium iodide, iodine, potassium hydroxide, triethanolamine and various compositions for 20 minutes at 22 ° C. The recovery of palladium, the composition of the solution and the concentration of beryllium in solution are specified in Tables 1-3.
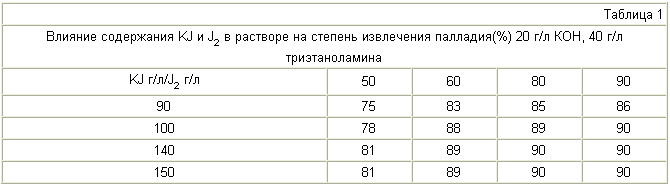
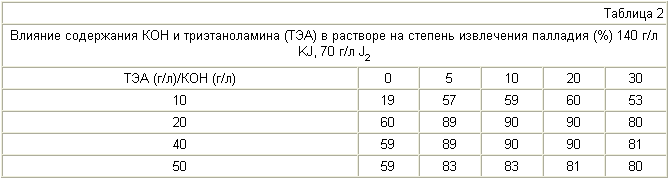

When the concentration of potassium iodide and iodine is less than 100 and 60 g / l of palladium extraction decreases (Table 1). Upper value of the specified ranges (150 and 90 g / l) of the above process is determined by a cost-effective implementation of border options. Triethanolamine at a concentration of less than 20 g / l and KOH in the absence of palladium extraction decreases. The same is observed at a concentration of triethanolamine and KOH, respectively, greater than 40 g / l and 20 g / l (Table 2). Beryllium content in the solution when using the proposed method is less than 0.04 mg / L and slightly changes with a solution of composition (Table 3).
At temperatures below 20 ° C palladium dissolution rate begins to decrease appreciably. Increasing the temperature of the solution above 40 ° C a noticeable increase in the rate of dissolution of palladium does not. Conducting the process at elevated temperatures is not economically feasible.
Compared to the proposed prior art method has the following advantages:
- selective dissolution of palladium is performed substantially preventing transfer of beryllium to the solution, moreover, the content of non-ferrous metals in the sludge slurry decreased to 2.3%;
- nonaggressive conduct the process in an alkaline medium;
- the process is carried out at a low temperature.
All this allows to drastically reduce the toxicity and corrosivity of process solutions obtained and vapors.
LITERATURE
Ginzburg SI, Gladyshevsky KA NA Ezerskaya et al., "Guidelines for the chemical analysis of platinum group metals and gold." M .: "Science", 1965, p.10.
RF patent №2140877, cl. C 01 G 55/00, 1999.
US Patent №4319923, cl. With 22 in 11/04.
RF patent №2085497, cl. C 01 G 55/00, C 22 B 3/00, 1997 (prototype).
CLAIM
The method of extraction of palladium from waste, comprising dissolving palladium and reduction, characterized in that the lead dissolving aqueous solution containing 100-140 g / l potassium iodide, 60-80 g / l iodine, 20-40 g / l triethanolamine and 5 20 g / l of potassium hydroxide.
A method according to claim 1, characterized in that the process is carried out at pH 7-11.
A method according to claim 1, characterized in that the process is carried out at a temperature of 20-40 ° C.
print version
Publication date 04.12.2006gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.