| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2097438
![]()
METHOD OF EXTRACTING METAL FROM WASTE
The name of the inventor: Buchikhin EP; Sysoev Yu.M .; Erisov A.G.
The name of the patent holder: Limited Liability Company "Company" Oriya "
Address for correspondence:
Date of commencement of the patent: 1996.05.29
The invention relates to a method for recovering materials from waste, for example the electronic industry, by iodide leaching.
SUMMARY OF THE INVENTION: chemical enrichment of wastes is carried out by successive extraction of heavy, non-ferrous metals and silver from them, and leaching of precious metals is carried out by the agitation method simultaneously with desorption of iodine from the plastic-ceramic base of enriched scrap, which is carried out by sequential treatment with slightly acid iodide iodide solutions An increased concentration of iodine to extract the bulk of gold, and then with a lower content of iodine for gold recovery, these solutions used in circulation after electrochemical gold separation, while generating the necessary amount of iodine, followed by washing scrap from potassium iodide and iodine bound to insoluble iodides Heavy and non-ferrous metals, are conducted by alkaline solutions, for example the production solution after the precipitation of gold, with the simultaneous conversion of insoluble metal iodides to hydroxides and subsequent aqueous washing carried out in combination with electrodialysis treatment of the reusable reusable.
DESCRIPTION OF THE INVENTION
The invention relates to the recovery of metals from waste and can be used in the processing of secondary raw materials, including the electronic industry, concentrates of the mining industry, etc.
A method for recovering metals from wastes is known (Autobus No. 1293070, class C 22 B 11/04) consisting in processing the previously prepared raw material with solutions of nitric acid or royal vodka, followed by the isolation of metals by the addition of reducing agents.
Disadvantages of this method are associated with the environmental hazard of the process, caused by the release of toxic gases and waste water, the irreversible loss of expensive reagents.
A method for recovering metals from an electronic scrap (US Patent No. 3,957,505, class C 22 B 11/04, 75/108) is also known, by treating it with an aqueous solution containing 10 parts by weight of water, 1 part of iodine, 0.7-20 parts Water-soluble iodide, the resulting solution is mixed with a reducing agent in the presence or absence of a buffer for reconstituting the dissolved gold iodide to metallic gold and precipitating substantially pure gold from the solution. After removing the precipitated gold from the aqueous solution, an oxidizing agent (hydrogen peroxide) is added to the latter. This leads to the restoration of the initial state of the solution, which is used repeatedly.
Disadvantages of this method are associated with irreversible losses of metals and expensive iodine with leached scrap by retaining the production solution in wet scrap and forming insoluble heavy metal iodides making up the bulk of the processed raw materials. At the same time, expensive reagents are irreversibly spent, the regenerated leaching solution is constantly diluted and saturated with extraneous anions, which in the future will require additional concentration and purification of solutions, without which the process of leaching of gold in the proposed regime will be impossible.
The most closely related to the proposed method is the method for recovering metals from waste, preferably the electronics industry (US Patent No. 5,026,420, class C 22 B 11/04, 75/712) including leaching of precious metals with iodide solutions containing 10 parts of water-soluble iodide and 1 part of iodine In the range of concentrations of 1-20 g / l, purification of production solutions after leaching from ions of dissolved metals, electrochemical gold evolution and regeneration of the leaching solution. At the same time, purification of iodide solutions obtained in the process of gold leaching from gold-bearing materials occurs by sorption extraction of dissolved metal ions on a strongly acidic cation-exchange resin, anions on a strongly basic ion-exchange resin. In addition, methods for precipitating hydroxides of metallic ions or conducting processes in a buffered medium are used to purify solutions. The process of simultaneous gold recovery and iodine regeneration is carried out, consisting in the fact that the production solution is supplied to the cathode compartment of the electrochemical cell where gold is electrochemically released at the cathode and the unreacted iodine is reduced to iodide and then from the cathodic compartment is fed to the anode where iodide is oxidized Up to iodine and thereby the reactivity of the leach solution is regenerated.
Disadvantages of the known method are incomplete washing of iodine from leached electronic scrap, where it is mainly contained in the form of water-insoluble iodides of heavy metals, possible losses of gold during washing by water because of the reduced solubility of the gold iodide complex in pure water, the formation of significant amounts of waste concentrates Ions of metals of regenerates, a rapid decrease in the activity of the leaching solution due to the interaction of the oxidant with heavy metals and copper, which make up the bulk of the processed raw materials.
Thus, large losses of expensive iodine with insoluble iodides of heavy and non-ferrous metals make this method not economical and restricts its use only for scrap with a low content of non-ferrous and heavy metals, which, in turn, narrows the scope of this method.
The object of the invention is to provide a method for extracting metals from wastes having an increased efficiency, excluding environmental pollution by toxic gases and waste waters and providing expansion of the raw material base for the production of precious, non-ferrous, rare and heavy metals.
The technological effect consists in reducing the losses of expensive iodine, increasing the cost-effectiveness, manufacturability of the method for extracting metals from the waste of the electronics industry.
The task is achieved by the fact that in a known method for recovering metals from waste, preferably the electronic industry (scrap), which includes treating the waste with iodide solutions, washing the leached product, purifying productive solutions after leaching from heavy metal ions, electrochemical gold evolution and regenerating the leaching solution, according to Of the invention, before chemical treatment, chemical enrichment is carried out by successive extraction of heavy, non-ferrous metals and silver from the waste, and the leaching of precious metals leads simultaneously with the desorption of iodine from the plastic-ceramic base of enriched scrap, which is carried out in two stages, on the first weakly acid iodide solutions with increased iodine concentration For the extraction of the bulk of gold, for the second with a reduced content of iodine for the recovery of gold, followed by the use of solutions obtained in circulation, after electrochemical gold extraction with the simultaneous generation of the required amount of iodine, the product is washed with potassium iodide and bound iodine with alkaline solutions, After the isolation of gold, with the simultaneous conversion of insoluble metal iodides to hydroxides and subsequent aqueous washing with subsequent electrodialysis treatment of the reused material, reused, with the leaching of metals being conducted by the agitation method. In addition, leaching can be conducted by agitation-percolation and percolation method.
Chemical enrichment, which is produced before leaching by successive extraction of heavy, non-ferrous metals and silver by electrochemical dissolution of tin, lead, aluminum and zinc in alkaline solutions with simultaneous isolation of lead-powder and subsequent purification of these solutions from aluminum and zinc Get an expensive commodity product in the form of a lead-limescale propolis and provides a closed process for alkali, thus saving the reagent.
Electrochemical dissolution of non-ferrous metals, iron and silver by means of copper-ammonia etching solutions or sulfuric acid solution, with simultaneous separation of copper at the cathode and subsequent cementation of non-ferrous metals and precipitation of silver chloride for re-use of pickling solutions and sulfuric acid, allows the marketable products in the form of copper , Silver chloride, ensuring the process is closed through copper-ammonia and sulfuric acid solutions, thereby eliminating spillway and saving chemical reagents.
The introduction of washing of leached waste (scrap) from captured gold with an iodide solution allows to reduce the irreversible losses of gold discharged from leached scrap and the fact that washing of scrap from captured iodine is carried out with alkaline solutions with simultaneous conversion of insoluble iodides to hydroxides followed by treatment with electrochemical and Electrodialysis method and the return of a concentrated solution of iodide salt to the reinforcement of the leaching solution, and a conduit for iodine washing allows minimizing iodine losses, organizing a closed circulation of washing solutions, eliminating the loss of an expensive reagent, organizing a closed cycle for water-soluble iodide salts used in leaching, In the complete absence of discharge of toxic waters into the environment.
Example
The material received for processing was a product obtained by mechanical enrichment of electronic scrap through crushing operations in a closed cycle, screening, air classification, followed by separation of the metal fraction by the method of magnetic and electrostatic separation in an amount of 100 g , containing, Au 0.52;
Ag 2.20;
Sn 15.9;
Pb 7.48;
Zn 0.36;
Cu 27.60;
Ni 0,20;
Fe 5.00 .
Separation of tin, lead, aluminum and a part of zinc from scrap was carried out by electrochemical dissolution in 10-20% alkali solutions, during anodic polarization of scrap. The leaching was carried out in an electrolytic cell with an anode in the form of a stainless steel basket into which scrap was loaded and a cylindrical cathode made of the same material, with constant mixing and removal of excess heat. The process was constantly monitored by changing the weight of the scrap. At a current density of 300 A / m 2 , a current strength of 4A , a ratio of solid to liquid (T: G) = 3 and (room) temperature of 50-70, the process ends in 6 hours , with the extraction of tin, lead and aluminum in the solution Zinc were, respectively (c) 92, 80, 65, 55 (the concentration of elements in the solution is 47.38, 20.2, 2.11, 0.67 g / l, respectively ). Simultaneously, the cathode was precipitated with a lead-acid powder gradually falling to the bottom of the cell. Current output at 18%
After washing the leached scrap from alkali, its weight was 75.6 g. The electrolyte was filtered from the lead-containing powder and reused. As the aluminum and zinc accumulate in the electrolyte, part of the solution is removed from the process and purified from them by precipitation of sodium aluminate by cooling the solution to 16-18 degrees and then cementing the zinc on the iron shavings. The purified electrolyte is returned to the head of the process.
Purification of gold-containing electronic scrap from copper and alkali-insoluble non-ferrous metals was carried out by electrochemical leaching of these elements into a copper-ammonia pickling solution or a monoethanolamine-based composition with the simultaneous release of powdered copper at the cathode. The process was carried out in the previously described electrolyzer with constant control of the weight loss of the loaded scrap. At a current density of 200 A / m 2 , T: G 2 , constant air purging and room temperature, the process ends in 6 hours , with the degree of recovery of copper, nickel and silver in the solution, respectively (c) 98, 90, 73 . The residual copper content in the solution after electrolysis is 10 g / l . After washing the leached scrap from the copper-ammonia pickling solution, its weight was 46.8 g .
The electrolyte was filtered from the copper powder and reused. As the silver and nickel accumulate in the electrolyte, part of the solution is removed from the process and, after distilling off the ammonia, silver chloride is precipitated from it and nickel is cemented. The solution is reinforced with ammonium salt, ammonia and returned to the head of the process.
Scrap removal from iron was carried out by the method of sulfuric acid leaching in the presence of an oxidizer, followed by precipitation of iron hydroxide.
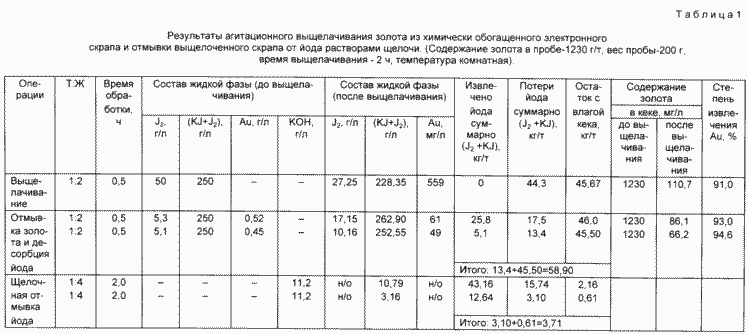
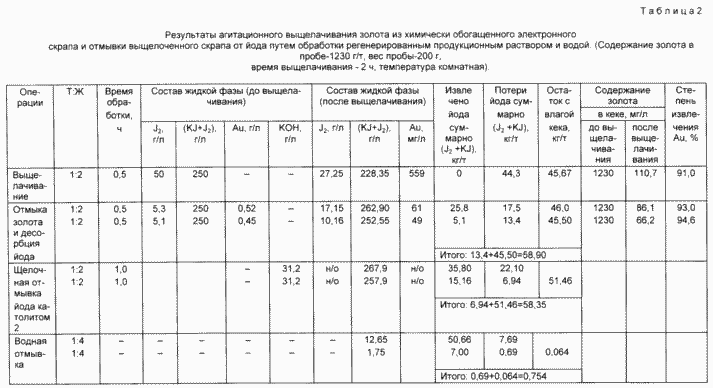
The material thus prepared (scrap) was first treated with iodine iodide containing a solution with a high iodine content ( 200 g / l KJ and 50 g / l J2 ), and then in two stages, a gold extraction and washing operation was carried out with simultaneous desorption of the sorbed on the plastic- Ceramic iodine base by treatment with iodine iodide solutions with reduced iodine content ( 200 g / l KJ and 0.1-5 g / l J2 ).
The production solution and the resulting washings were electrochemically processed in a multi-compartment electrolyzer at different feed rates with the production solution and wash water.
The process was carried out in a four-chamber membrane cell. Washing water was introduced into the first cathode chamber, gold was recovered and iodine was washed off. In an adjacent anode chamber, iodine was oxidized to a concentration of 5 g / l and the solution was returned to a gold washing operation (anolyte 1).
The production solution was introduced into the next cathode chamber, gold was recovered, and unreacted iodine was reduced to iodide, after which the solution was sent to the anode chamber, where iodide was oxidized to iodine ( 50 g / l ). Due to the electrochemical reaction in the cathode chamber, the solution is alkalinized to pH 13 . Part of the catholyte was sent for regeneration and then for leaching (catholyte 1), the other part (catholyte 2) for the recovery of iodine captured with insoluble iodides of metal impurities by converting them into hydroxides. The resulting washings with iodine were combined with the production solution and again fed into the cathode chamber of the cell.
Experiments on iodine washing were carried out as follows: a sample of the raw material ( 200 g ) was leached with an iodine iodide solution of 200 g / l KJ and 50 g / l J 2 at G: T 2 and a leaching time of 30 min , and then washed with gold solution With an iodine content of 5.3-5.1 g / l and a total content of J and J 2 equal to 250 g / l (anolyte 1) in 2 stages at G: T 2 and a mixing time of 30 min.
The filtered washed gold scrap (cake) with a moisture content of 20% was divided into two equal parts ( 100 g solid). One part was washed with a solution of 0.1 N KOH , a second catholyte 2.
The final washing of the cake from potassium iodide was carried out by the method of displacement by water in two stages. The washing water was sent to an electrodialyzer at the outlet of which a brine with a concentration of 120-140 g / l was obtained in potassium iodide and desalted water directed to the washing operation.
The results and conditions of the experiments are shown in Table. 1 and 2 .
CLAIM
A method for recovering metals from waste, comprising leaching of waste with iodide solutions containing iodine, washing the leached product, electrochemical separation of gold from the production solution, regeneration of the leach solution and its use in leaching, characterized in that before chemical leaching, chemical enrichment is carried out by successive extraction of heavy, Ferrous metals and silver, and leaching is carried out in two stages: on the first weakly acidic iodide solutions with a high concentration of iodine to extract the bulk of gold, for the second with a reduced content of iodine for the recovery of gold, followed by the use of the obtained solutions in circulation after electrochemical gold extraction and simultaneous generation Of the required amount of iodine, washing of the product from iodide and bound iodine is carried out with alkaline solutions with the simultaneous conversion of insoluble metal iodides to hydroxides, subsequent aqueous washing and electrodialysis treatment of the reused waste.
The method according to claim 1, characterized in that the leaching is conducted by an agitation method.
The process according to claim 1, characterized in that when the leached product is washed as an alkaline solution, a regenerated production solution is used.
print version
Date of publication 04.12.2006гг




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.