| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2116362
![]()
Method of extracting precious metals from spent catalysts
Name of the inventor: Aleev RS .; Cemil U.M .; Dalnova YS .; Borisova VV .; Poloumov AV .; Yesipenko AI .; Andrianov VM .; Kalimullin AA
The name of the patentee: Institute of Catalysis and petrochemical plant with an experienced Academy of Sciences of Bashkortostan
Address for correspondence:
Starting date of the patent: 1997.04.01
The method may be used for extraction of metals from the catalysts. , The processing of spent catalyst a freshly prepared mixture of hydrochloric acid and concentrated hydrogen peroxide in static or dynamic modes of circulation at a temperature of 0 to 100 o C and atmospheric pressure. The filtered solution was combined with the solution obtained by washing the filter cake with water. The combined solution containing the precious metals, the sorbent treated - 1-hydroxy-2- (perhydro-1,3,5-dithiazine) -5-yl-ethane in an amount of 5 - 10 N per 1 g of the extracted metal at a temperature of 0 - 100 o C atmospheric pressure for 2 - 10 hours to complete absence of traces of precious metals in solution. The proposed sorbent has a high adsorption capacity with respect to the metal to be extracted is reduced to 10 - 12 times the reagent consumption, a high degree of treatment - 95 - 98% of precious metals from spent catalysts.
DESCRIPTION OF THE INVENTION
The invention relates to hydrometallurgy, and in particular to methods for recovering metals from spent catalysts.
Known hydrometallurgical processes extracting precious metals from spent catalysts.
The main stages are known processes:
- dissolving metals using an oxidant in acidic medium;
-
the most effective system:
chlorine in hydrochloric acid solution;
[1, 2, 3];
-
extracting precious metals from a solution by using m-precipitating reagents. d .; [4, 5, 6];
the allocation of precious metals from the reagent concentrate - roasting sludge etc. [4, 5, 6].
Dissolving metal by methods 1-3 are equally effective, but when used as oxidizer nitric acid, the process including toxic wastes appear trudnoutiliziruemye and nitrogen oxides; the use of pressurized chlorine requires special attention to the issues of safety and high production standards; It combines the most environmentally friendly and the processability in the case of a mixture of hydrochloric acid and hydrogen peroxide. In this embodiment, the process can be conducted under relatively mild conditions and is characterized by the absence of gas emissions.
Extracting precious metals from a solution by using precipitants such as hydrazine or other reducing agents leads to the formation of fine, trudnoulavlivaemogo to precipitate filters. Extraction with organic solvents precious metal compounds then requires additional stripping operations, which increases the value of losses of precious metals; and the latter applies to the use of ion exchangers.
Reducing the loss of precious metals allows for the reception, applied by the company "Rhone-Poulenc Industries" [9] of precious metals saturated sorbent-exchanger is not subjected to a reverse exchange, and burn, reducing the number of operations and getting just pure metal.
Process closest in technical essence to the claimed one comprises the steps of dissolving the essence to the claimed one comprises the steps of dissolving the precious metals of acid-oxidant mixture, extracting precious metals from a solution by adsorption on an anion exchanger "Duolite A 101 D" and combustion concentrate sorbent a temperature of 880 o C to yield pure metals. The process provides a high degree of extraction of metals by reducing the number of operations.
Disadvantage of this method is the high consumption-ion exchanger sorbent per unit mass of metal to be extracted as a sorbent is irretrievably lost when burned.
The reason for the large sorbent consumption is the low capacity of the sorbent with respect to extractable metals: up to 50 g per 1 g of the precious metal.
The authors proposed a method of reducing sorbent consumption due to capacity with respect to the recoverable precious metals.
The task is carried out using as the sorbent 1-hydroxy-2- (perhydro-1,3,5-dithiazine) -5-yl-ethane (hereinafter OPDE).
Application OPDE sorbent consumption reduces during 5-10 g per 1 g of the extracted metal.
The suggested process of extracting precious metals from spent catalysts, comprising the steps of:
- dissolving a mixture of precious metals with hydrochloric acid and hydrogen peroxide;
- sorption extraction of precious metals from a solution using 1-hydroxy-2- (perhydro-1,3,5-dithiazine) -5-yl-ethane;
- the allocation of precious metals from concentrate sorbent ashing at 600-650 o C.
The proposed process achieves high recovery rates (95-98%) of precious metals from spent catalysts based on silica, alumina.
The process was conducted as follows: the spent catalyst was treated with a freshly prepared mixture of conc. hydrochloric acid and hydrogen peroxide (30% solution & Shipping) in proportions (by weight) of from 5: 1 to 1: 2, in amounts of from 1.2 g to 3.6 g of a mixture of the catalyst mass, in static or dynamic modes of circulation, at temperatures from 0 to 100 o C and atmospheric pressure.
The filtered solution was combined with the solution obtained by washing the filter cake with water in an amount of from 1.0 g to 4.8 g per 1 g of the catalyst.
The washed cake contains no precious metals.
The combined solution containing the precious metals, the sorbent treated -1 -hydroxy-2- (perhydro-1,3,5-dithiazine) -5-yl-etananom in an amount of 5-10 g per 1 g of the extracted metal at a temperature of 0-100 o C and atmospheric pressure, in static or dynamic mode in a state for 2-10 hours. to no traces of precious metals in the solution.
The filtered and washed with water sorbent precipitate concentrate is dried and calcined in an oven at a temperature of 600 - 650 o C, was obtained in the extracted metal reconstituted form in a yield of 95-98%.
A distinctive feature of the process is used as a sorbent 1-hydroxy-2- (perhydro-1,3,5-dithiazine) -5-yl-ethane to extract gold and platinum group metals from solution.
Advantage OPDE sorbent before the sorbent known "Duolite A 101 D" in the process of extraction of precious metals is significantly higher sorption capacity with respect to the recoverable metals reaching 0.1 -0.2 g of metal per 1 g of the sorbent mass. Increased capacitance entails a reduction in reagent consumption of 10-20 times compared to the rate of sorbent "Duolite A 101 D".
The present invention illustrates an example
example 1
Weigh 5 g of the catalyst containing 0.22% gold and 0.56% of palladium were placed in a column type reactor provided at the bottom of the filtrate and stopcock disposed below the filter and the valve is closed when the reactor was added a mixture of 6 g of 30% strength solution of hydrogen peroxide and hydrochloric acid, taken in equal amounts. The reaction mixture was allowed to stand at room temperature for 8 hours, after which the bottom valve was opened and the solution was poured into 2 N reactor, which is a container with a stirrer and a filter at the bottom. N remaining in the reactor 1 the solid phase was washed with water three times by filling the reactor with water and then draining the washing water and N 2 into the reactor, and then the washed solid phase was discarded.
N 2 into the reactor, a solution containing gold and palladium were added 390 mg of 1-hydroxy-2- (1.3,5-perhydro-dithiazine 5-) yl-ethane at room temperature, stirred for 6 hours after which the precipitate was filtered off, the precipitate was washed on the filter with water until neutral wash water, dried and calcined in a muffle furnace at 600 o C for 2 hours. The sol contained 10.5 mg of gold, palladium 272 m (yield respectively 98.5% gold and 97.1% of palladium).
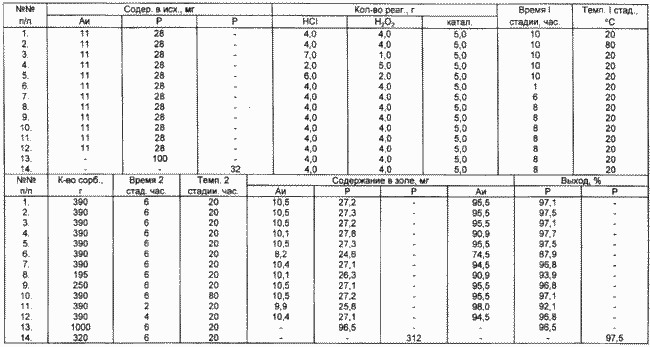
The results of other Examples are shown in the table.
CLAIM
Method of extracting precious metals from spent catalysts comprising precious metals dissolution of acid-oxidant mixture, the sorption of precious metals from the solution and isolating them from the sorbent ashing concentrate, characterized in that as a sorbent is used 1-hydroxy-2- (perhydro-1,3 5-dithiazine) -5-yl-ethane.
print version
Publication date 07.11.2006gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.