| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2100484
![]()
METHOD OF OBTAINING SILVER FROM ITS ALLOYS
The name of the inventor: Lebed AB; Skorokhodov VI; Naboichenko SS; Mastyugin SA; Khusainov F.G.
The name of the patent holder: Open Joint-Stock Company "Uralelectromed"; Ural State Technical University
Address for correspondence:
Date of commencement of the patent: 1996.02.14
Use: refers to the production of silver in the refining of silver alloys, while simultaneously increasing the recovery of the noble metal. SUMMARY OF THE INVENTION: The method comprises dissolving the parent alloy in nitric acid in the presence of ammonium ions, separating the slurry, treating the heated solution of silver nitrate with ammonium hydroxide after sorption cleaning with an anionite, filtering the hydroxides of the impurity metals, and after electrolysis of silver from the silver nitrate solution, the electrolyte is returned to dissolve the alloy . The method allows one-stage production of cathode silver, to increase the direct extraction of silver, to eliminate liquid waste and anode residues, and to process silver alloys with an silver content of less than 95% on an industrial scale.
DESCRIPTION OF THE INVENTION
The invention relates to non-ferrous metallurgy, refining of silver alloys and can be used in refining industries.
The most common electrolytic methods of refining, which make it possible to obtain high purity silver, and transfer impurities to intermediates, from which valuable components are then extracted in separate ways.
However, electrolysis is considerably complicated by the presence of significant amounts of copper, tellurium, palladium and other platinoids in the initial silver alloys.
A method for the electrolytic refining of a silver-gold alloy (Dore alloy) containing not less than 95% silver, not more than 3% gold, and up to 2% copper, lead, nickel, iron, tellurium, platinum and palladium (in total) is known. An electrolyte solution is a silver nitrate solution (up to 60 g / dm 3 Ag), and the anode is a refined alloy. The cathode current density is 300 A / m 2 . In all cases, the anode and cathode spaces are separated.
During the electrolysis, the copper content of the electrolyte is controlled. When the concentration of copper in the electrolyte is above 45 g / dm 2, the electrolyte is withdrawn for processing, cementing silver and other noble metals that have passed into the solution. The next batch of electrolyte is prepared from the already obtained silver, dissolving it in nitric acid [1]
Disadvantages of the method:
1. The method involves processing alloys with a silver content of at least 95%
2. The method does not provide regeneration of the electrolyte. The contaminated electrolyte is recycled separately.
3. The formation of anode residues and products of processing of substandard electrolyte leads to a reduction in direct silver recovery of not less than 10%
A method of electrolytic refining of silver is also known [2]
In this method, the electrolyte is prepared by dissolving the original silver-gold alloy in a solution of nitric acid (at low palladium and tellurium contents in the alloy) or cathode silver. Electrorefining of the initial silver alloy is carried out in a solution of silver nitrate with addition of nitric acid with a soluble anode before the accumulation of a critical impurity content in the electrolyte (Cu 80 100 g / dm 3 , Te 16 30 mg / dm 3 , Pd O, 1 0.2 g / dm 3 ), after which the impurities pass into cathode silver. With the accumulation of impurities, the electrolyte is periodically removed for processing with the liberation of silver and precious precious metals.
When processing silver alloys with a high content of tellurium and platinoids for the production of silver grade Cp-A1, re-electrolysis of cathode silver is required.
Disadvantages of the method:
1. The method does not allow the use of the original gold-silver alloy for the preparation of the electrolyte with tellurium content of up to 0.1-0.15% and palladium up to 0.25-0.35%, and the volume of return of the cathodic silver obtained for the preparation of the electrolyte is up to thirty%
2. A significant amount (up to 30%) of precious metals remains in the incomplete production solutions, which requires additional processing. The method does not provide regeneration of the electrolyte.
3. Direct extraction of silver is not higher than 70% due to the formation of anode residues and waste electrolytes.
4. Multi-stage refining is necessary before the conditioning product is obtained.
The closest invention in terms of technical essence is the method of electrolytic isolation of silver from nitric acid solutions obtained after leaching anode copper slimes with nitric acid.
The excess acidity of the nitric acid solutions obtained before the electrolytic release of silver is first reduced by diluting the solution and then partially neutralizing the acid with 5% solutions of sodium hydroxide and ammonium hydroxide [3]
Disadvantages of this method:
1. The method according to the prior art is applicable to anode slurries, which are more complex in chemical and phase composition than silver alloys.
2. Dissolution of anode copper slimes in a single nitric acid inevitably leads to the release of nitrogen oxides, including by dissolving concomitant non-ferrous metals and their compounds. (This aggravates the environmental situation in the industrial implementation of the method).
3. The prototype method does not provide for the rotation of working solutions, which reduces the degree of direct extraction of noble and rare metals up to 70% and leads to inevitable irreversible losses of noble metals with withdrawable solutions.
4. The method does not guarantee obtaining a conditioned (99.99% purity) silver, especially for the content of platinum and tellurium. The method can not guarantee the production of silver that meets the requirements of GOST, which will require additional processing of the silver produced by this method.
The invention solves the problem of one-step refining of silver alloys with an increased copper content (up to 2.0%), the amount of platinoids to 0.4% tellurium up to 0.2%, and allows to increase the direct extraction of silver, to eliminate re-electrolysis in obtaining high grades of the desired product.
This is achieved by the fact that all operations are performed in the presence of ammonium ions: the initial silver alloy is dissolved in nitric acid in the presence of ammonium ions to a concentration of 3.0 5.0 g / dm 3 , the precipitate is separated, the solution is sorptively purified from platinoids, and the filtrate is treated Ammonium hydroxide, the precipitate is filtered off and electrolytically precipitated by silver, and then the electrolyte is returned to dissolution of the parent alloy.
A comparative analysis of the known technical solutions and the claimed invention allows to conclude that the invention is unknown from the state of the art and corresponds to the criterion of "novelty". From the prototype, the inventive method is characterized in that all the operations of the process are conducted in the presence of an ammonium ion, and the electrolyte obtained by dissolving an initial silver alloy in an acid containing 3.0 5.0 g / dm 3 of an ammonium ion is subjected to electrolysis sorption purification on an anionite from platinoids , Then it is treated with ammonium hydroxide and filtered.
The essence of the claimed invention for a specialist engaged in silver refining should not be explicitly known from the prior art; The claimed method of obtaining silver allows one to obtain the desired product in one step with the purity of cathode silver, which meets the requirements of GOST 28595-90 to obtain silver grade not lower than Cp-A1, to increase the direct extraction of silver up to 95%, to exclude liquid wastes that need to be disposed of and anode residues. The method makes it possible to process silver alloys with a silver content of less than 95%
Modes of the implementation of the method are chosen experimentally.
Dissolution of the original silver alloy (production of the electrolyte) is carried out in a sealed reactor without stirring in a solution of nitric acid under the pressure of the evolving gas phase in the presence of ammonium ions. The ammonium ion concentration in the final solution was determined experimentally at the stage of dissolution of the initial alloy 3.0 5.0 g / dm 3 . The concentration of ammonium ions is less than 3.0 g / dm 3 , resulting in an increased concentration of toxic nitrogen oxides in the off-gas phase.
(Table, item 1).
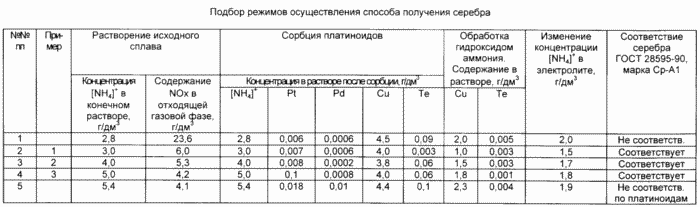
The concentration of ammonium ions in the initial solution of silver nitrate above 5.0 g / dm 3 does not lead to a decrease in the concentration of nitrogen oxides, but leads to a deterioration in the sorption purification of the solution from platinoids.
(Table, item 5).
The concentration of ammonium ions in the initial solution of silver nitrate in the range 3.0 5.0 g / dm 3 provides the content of toxic nitrogen oxides in the effluent gas phase is not higher than 6.5 g / m 3 , and in the solution leaving after sorption the content of platinum and palladium is not More than 0.01 and 0.005 g / dm 3, respectively.
This ensures that the content in the final product of the amount of platinum is not more than 0.001%, i.e. Compliance with the requirements of GOST 28595-90.
(Table, items 2, 3, 4).
After sorption on the anionite of platinoids, the nitric solution of silver is treated with ammonium hydroxide, controlling the copper content to a residual concentration of 1.5 2.0 g / dm 3 and tellurium to 0.003 g / dm 3 .
(Table 1, items 2, 3, 4).
Higher levels of copper and tellurium do not allow you to obtain the silver required by GOST quality.
(Table, item 5).
The method is tested on a semi-industrial scale when processing a silver-gold alloy produced by JSC "Uralelectromed" composition, wt.
- Au 4.9 6.4
- Ag 91 92
- Pf 0.09 0.1
- Pd 0.31 0.33
- Te 0.05 0.33
- Cu 0.84 3.65
And is illustrated by examples of practical implementation of the method.
Example 1 . A portion of the silver-gold alloy is dissolved at 910.degree. To 110.degree . C. in a solution of nitric acid in the presence of ammonium ions under a pressure of a liberating gas phase of 0.05 MPa to a silver concentration in the final solution of 150 g / dm.sup.3 and ammonium ions of 3.0 g / dm.sup.3. The maximum content of nitrogen oxides in the off-gas phase was 6.4 g / m 3 .
The sludge is filtered off, and the nitric acid solution is passed through the anion exchanger at a rate of less than 3.0 v / h.
The solution after the sorption column contains 0.007 g / dm of platinum, 0.0006 g / dm 3 of palladium, 4 g / dm 3 of copper and 0.07 g / dm 3 of tellurium.
The silver nitrate solution is heated to 50 ° C. and ammonium hydroxide is added to a content of 1.5 g / dm 3 in the copper solution. The precipitate of the hydroxides is filtered off after settling.
The hydrated cake contains, wt. Silver 26, copper 31, tellurium 0.6. Then a solution of silver nitrate is placed in an electrolyzer and electrolyses the silver at a current density of 500 A / m 2 and 20 ° C to a silver content of 50 g / dm 3 in the electrolyte. Electrolyte and cathode silver are removed from the electrolyzer, the electrolyte is returned to the dissolution operation, and the cathode silver is washed, dried, melted, and analyzed. Silver corresponds to GOST 28595-90 for the brand Cp-A1.
Example 2 . The process is carried out as in Example 1, dissolving the parent alloy in nitric acid to a concentration of ammonium ions of 4.0 g / dm 3 . The maximum content of nitrogen oxides in the effluent gas phase is 5.3 g / dm 3 . Ammonium hydroxide solution of silver nitrate is processed to a content of 1.5 g / dm 3 in copper solution, 0.003 g / dm 3 tellurium. Cathode silver after electrolysis corresponds to GOST 28545-90 grade Cp-Al.
Example 3 . The process is carried out as in Example 1, dissolving the parent alloy in nitric acid in the presence of ammonium ions to a concentration of 5.0 g / dm 3 . The maximum content of nitrogen oxides in the off-gas phase is 4.2 g / dm 3 . The solution after sorption of impurities is treated with ammonium hydroxide to a copper content of 1.8 g / dm 3 , tellurium 0.001 g / dm 3 . After electrolysis cathode silver corresponds to GOST 28545-90 grade Cp-Al.
Advantages of industrial use of the claimed method
Positive results of the test of the method in the operating conditions of OJSC "Uralelectromed" allow considering the claimed method of obtaining silver from its alloys industrially applicable.
1. The method provides a one-stage preparation of pure silver by electrolysis at elevated impurity levels.
2. The method provides complete regeneration of the electrolyte.
3. Formation of anode residues and liquid intermediates is eliminated, direct extraction of silver is increased.
4. Direct extraction of silver is increased by no less than 10%
5. The method makes it possible, on an industrial scale, to process silver alloys with a silver content of less than 95%
CLAIM
A process for the preparation of silver from its alloys, including dissolving the nitric acid solution of the parent alloy, separating the insoluble residue, neutralizing the solution using ammonium hydroxide, and electrolytically recovering the silver from the nitric acid solution, characterized in that dissolution of the initial alloy in nitric acid is conducted in the presence of ammonium ions to a concentration thereof Solution 3 5 g / dm 3 , after which the solution is subjected to sorption cleaning, then the filtrate is treated with ammonium hydroxide, and after separation of the precipitate, an electrolytic precipitation of silver is carried out with the return of the electrolyte to dissolve the initial alloy.
print version
Date of publication 14.03.2007gg




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.