|
Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2114926
![]()
LEACHING GOLD WITH HELP
NITROGEN AND SULFUR-CONTAINING HETEROCYCLIC AROMATIC COMPOUNDS
The name of the inventor: Kristiansdottir Sigraydew Soul (US); Thompson Jeffrey Scott (US)
The name of the patentee: EI Du Pont De Nemours and Company (US)
Address for correspondence:
Date of commencement of the patent: 1994.10.06
The method can be used in processes requiring the dissolution of metallic gold, especially when hydrometallurgical extraction of gold from ore. The process of dissolution of metallic gold is carried out in a leaching system containing a ligand and an oxidizer, where the solubility of gold is increased by the addition of aromatic compounds containing sulfur or nitrogen in the ring. Gold leaching is carried out using less corrosive-aggressive solutions without significant losses in the dissolution rate of the metal.
DESCRIPTION OF THE INVENTION
The invention relates to the field of dissolution of metallic gold by a leaching solution, in particular hydrometallurgical extraction of gold from ore. In particular, this applies to the gold leaching region, where a ligand and an oxidizing agent are used for leaching, as a dissolution activator, a heterocyclic aromatic compound containing nitrogen or sulfur in the ring is used.
Many processes require the dissolution of gold, for example, the extraction of metallic gold from ore containing small amounts of metal, the liberation of gold from gilded objects and the production of soluble gold compounds for use in the production of catalysts. However, gold as a metal is known for its resistance to dissolution and chemical interaction. For example, gold will dissolve in aqueous solution only if the solution contains a suitable ligand or binding agent for gold plus an oxidant. None of the components individually is not effective. This combination of a ligand and an oxidizer is called a leaching system.
In the last century, many leaching systems have been proposed and used for gold, mainly for gold mining. The most widely used system is the combination of sodium cyanide as a ligand with air (oxygen) as an oxidizer, mainly because of the economy and simplicity of the process. Other important ligand / oxidant systems are thiourea and thiocyanate with ferric ions, sodium thiosulfate and air, copper (II) sulfate and ammonia, sodium chloride and sodium hypochlorite, sodium bromide and bromine, as well as many other systems. The chemistry of these and other alternative leaching systems is described in a review article by JB Hiskey and VPAtluri, Mineral Processing and Extractive Metallurgy Review, 4,95-134 (1988). The choice of a particular leaching system depends on various factors, including the cost of components, safety, environmental protection and corrosion of equipment. For mining processes, the main factor in choosing a particular leaching system is often the ease of separating impurities associated with gold in the ore.
It is known that some divalent metal cations, such as lead, mercury, thallium and bismuth, accelerate the dissolution of gold in cyanide solutions. The mechanism of action is unknown, despite the fact that it involves depolarizing the surface of gold and preventing the passivation of the gold surface. The use of these metals is undesirable. If they are allocated with gold, further expensive cleaning may be required. If any part of these highly toxic metal ions gets into waste from ore processing, they will create a serious and long-lasting danger of environmental pollution.
The improvement of any of the commonly used leaching systems will be of great importance, especially if it is widely used in a variety of systems. This improvement could give a higher yield of dissolved gold under standard dissolution conditions, shorten the cycle time at this stage, reduce operating rigidity, use lesser amounts of liquor components, or lead to other methods of reducing cost, increasing safety, or increasing gold yield. At present, neither a method for improving the operation of commonly used leach systems without the use of poisonous metal compounds is open, nor a method that is widely applicable.
US Patent 3,597,290 discloses a method for chemically dissolving metals and, in particular, describes etching of copper with acidified hydrogen peroxide. The method uses a solution containing a strong organic acid or an inorganic acid (other than hydrohalic acids) plus hydrogen peroxide and a saturated lower aliphatic alcohol. This system may optionally contain catalytic amounts of a metal salt with a lower oxidation potential than that of the metal to be dissolved, to enhance the dissolution of the latter, catalysts, including salts of metals such as silver, mercury, palladium, gold and platinum. Optionally, the system can and include nitrogen compounds having at least one copper bonding site, such as urea, pyridine, amines and acid amides. In contrast to the present invention, the '290 patent does not provide a method for enhancing the dissolution of gold or other metals, but rather suggests the use of catalytic amounts of salts of certain noble metals to enhance the dissolution of other metals with a higher oxidation potential, such as copper. Said nitrogen-containing compounds are more optional than necessary and are useful as copper-binding agents for suppressing copper pruning in a selective etching process.
The Polish patent PL 130.801 discloses a process for the recovery of silver and copper from sulphide ores, including the use of a mixture comprising pyridine and its hydrochloride to dissolve minerals containing these metal ions, separating the inert material by filtration, adding water, extraction with chloroform to remove silver chloride and pyridine and reducing Copper from the aqueous phase by electrolysis. The process is similar to many examples described in the literature, where nitrogen-containing heterocyclic compounds are used to convert metal complexes to an organic solvent. The '801 patent provides for increasing the efficiency of the gold leaching system containing the ligand and oxidizer by adding catalytic amounts of an aromatic heterocyclic compound containing nitrogen or sulfur in the ring. It rather involves the use of large amounts of pyridine and pyridine hydrochloride to dissolve the sulfide-containing silver and copper ores and to separate copper ions from silver ions by extraction of silver and pyridine into the chloroform solution.
US Patent No. 5,169,503 discloses a process for extracting metals from an ore using a leachate system consisting of chloride, hypochlorite, and optionally cyanuric acid, a nitrogen-containing heterocyclic compound. Cyanuric acid is used to prolong the life of the hypochlorite solution under acidic conditions, even if it is determined that the actual reaction rate between the metals to be dissolved and the chlorine-containing compounds is reduced.
Numerous examples in the literature describe the use of nitrogen-containing heterocyclic aromatic compounds in combination with soluble gold compounds for various purposes. US Patent 4,654,445, Japanese Patent 01111824; Z. Zhang and W. Gam, Huaxue Shiji, 137-139 (1982) and M.Igbal and M. Ejaz, Radiochim. Acta, 22, 37-39 (1975). For example, a common application for extracting gold from aqueous solutions of their salts by solvent extraction. These nitrogen-containing aromatic heterocyclic compounds are used in the preparation of resins to separate soluble metal species from solutions and are often used to improve the deposition of gold plating, allowing the use of high current density without loss of current output. As the closest analogue it is recommended to use the patent EP, 0358004, C 22 B 11/08, publ. 14.03.90 a method for dissolving gold and a ligand / oxidizer leaching system, comprising adding to the system a catalytic amount of a catalytic compound. In all of these examples, heterocyclic aromatic compounds react with soluble metal ions and do not participate in the dissolution of the metal, in fact, in the case of electroplating, the process is just the reverse, removing the metal from its solution.
Thus, a method is needed to increase the efficiency of the leaching system for dissolving gold, which would increase the rate of dissolution, make possible a higher yield of the metal, use fewer reagents or allow less stringent conditions. In particular, a catalytic method is needed to improve such leaching systems that are widely applicable to a large number of systems and that do not require the use of salts of toxic metals.
This invention provides an improvement in the leaching of metallic gold in systems using a ligand and an oxidizing agent. The improvement includes the addition of catalytic amounts of optionally substituted heterocyclic aromatic compounds containing sulfur or nitrogen in the ring, provided that sulfur and / or nitrogen heteroatoms and heterocyclic ring in such heterocyclic compounds are available to form a coordination bond with the surface of the undissolved metal gold.
In traditional systems for leaching gold, an oxidizer is used to first convert metallic gold to a gold ion and a ligand that forms a coordination bond with the gold ion formed.
In the improved system of the invention, a nitrogen-containing and / or sulfur-containing aromatic heterocycle is added which promotes the dissolution of gold at a higher rate or under milder conditions than without it. The resulting gold complex consists of an oxidized metal ion, connected by a coordination bond with the added ligand and, possibly, solvent molecules; An aromatic heterocyclic compound is not part of the coordination sphere and is not consumed in the process.
In Fig. 1 shows the effect of various heterocyclic compounds of the invention on the dissolution of gold in a sodium chloride-sodium hypochlorite system; In Fig. 2 - the effect of pyridine on the dissolution of gold in the sodium bromide system - sodium hypobromite; In Fig. 3 - dissolution of gold in the ammonium copper-thiosulphate-air system in the presence or absence of pyridine; In Fig. 4 - efficiency of leaching of gold from gold-bearing ore with sodium cyanide-air solution in the presence or absence of 5 ppm N-methylimidazole.
All metal leaching systems commonly used today require the addition of a ligand to bind a metal ion and an oxidant to oxidize the metal. For metals such as gold, strong oxidants, such as chlorine or bromine, are required, and many ligands, such as chloride or bromide ions. Such leaching solutions are highly corrosive and should be used in expensive equipment that is inert to these substances. And such strong oxidizing solutions are often not selective in oxidative reactions, which leads to undesirable contamination of the desired metallic product.
The addition of activator compounds such as those described by Applicants allows the use of less corrosive-aggressive metal leaching solutions without significant losses in the dissolution rate of the metal. Such an activator allows the use of a smaller amount of reagents for the dissolution of metals. Not only is less ligand required to dissolve the metal at an acceptable rate, but also a smaller amount of reagent for yield and / or decomposition at the end of the leaching cycle. In a mining industry using, for example, sodium cyanide, the use of smaller amounts of cyanide means the use of smaller amounts of a cyanide decomposition reagent at the end of the leaching cycle. In addition, a lower concentration of cyanide in the reservoir for waste in a pond with a rich solution and other aqueous solutions means a reduction in the risk of environmental pollution. Thus, the present invention provides significant advantages for the mining industry.
The claimed invention contemplates the process of increasing the dissolution or leaching of metallic gold by adding catalytic amounts of certain heterocyclic aromatic compounds containing nitrogen and / or sulfur in the aromatic ring to the ligand / oxidant leachate system.
In accordance with the objectives of the present disclosure of the claimed invention, it is meant that the following terms have the meanings given below.
The word "leach" means "to extract or dissolve the component that forms part of the solid mixture." The leaching solution or system is a solution or system containing the components necessary to extract the desired component. In particular, as used by the Applicants to describe the dissolution of gold, the leaching system contains both an oxidizing agent that oxidizes metallic gold to a gold cation and a ligand to form a coordination bond with the gold cation.
Using the phrase "catalytic amounts" or "catalytic compound" for certain heterocyclic compounds. Applicants have in mind the traditional meaning of the word "catalytic", i.e. Heterocyclic compounds do not change during the leaching process. In particular, Webster's Ninth New Collegiate Dictionary defines the term "catalysis" as "a change and, mainly, an increase in the rate of chemical reaction caused by a substance that does not chemically change until the end of the reaction." Typically, the catalyst is used in less than stoichiometric amounts.
Using the expression "leaching system using a ligand and an oxidizer". Applicants include cyanide and air or dissolved oxygen, chloride and hypochloride; Bromide and bromine; Iodide and iodine; Thiocyanate and ferric ions; Thiourea and ferric ions; Ammonium thiosulfate, copper, ammonia and air or dissolved oxygen; Malononitrile and air or dissolved oxygen; And cyanide, chloride, bromide, iodide, thiocyanate, thiosulfate, malononitrile and thiourea on a gold electrode in anodic gold dissolution.
In the description of "nitrogen and sulfur-containing aromatic heterocyclic compounds" of the invention. Applicants include those compounds in which at least one nitrogen and / or sulfur heteroatom in the ring is available to form a coordination bond with the surface of the solid metal during the dissolution process. In particular, aromatic heterocycles containing nitrogen and / or sulfur in the ring are included, in which
1) the nitrogen atom in the ring is not protonated;
2) at least one sulfur or nitrogen atom in the ring has no substituents;
3) a steric obstacle does not interfere with the heteroatom or the aromatic structure containing it, form a coordination bond with the surface of the solid undissolved metal. A sterile obstacle can be associated, for example, with the presence of large substituents on the ring, which prevent the approach of either the heteroatom or the heterocyclic ring to the metal surface.
HETEROCYCLIC COMPOUNDS OF THE INVENTION
The heterocyclic compounds of the invention are effective in a wide range of leaching systems typical of gold mining processes, especially in sodium cyanide-air systems; Sodium chloride - hypochlorite; Sodium bromide-bromine; Copper - ammonium thiosulfate; Ferric thiocyanate ion; Thiourea and ferric ion; Iodide and malononitrile. However, as described below, not every proposed additive can be equally effective in each leach system due to the likelihood of side chemical reactions or the properties of certain leach solutions (pH, solution potential, ligand, gold, etc.). And Applicants believe that the heterocyclic aromatic compounds of the present invention will act not only as activators in various leaching systems commonly used in gold mining processes but will also be applicable to other processes requiring gold dissolution.
The heterocyclic aromatic compounds of the present invention have several common properties. They all contain one or more nitrogen and / or sulfur atoms in the heterocyclic aromatic ring, where a nitrogen or sulfur heteroatom is available to form a coordination bond with the metal surface. The heteroatom should have a free electronic pair for coordination. Nitrogen-containing compounds should not have steric hindrance, and the pH of the leach solution should be sufficiently alkaline to prevent protonation of the nitrogen heteroatom. Examples of suitable nitrogen-containing heterocyclic aromatic compounds include pyridine, imidazole, N-methylimidazole, 2,2'-dipyridyl, 2,3-, 2,4-, 3,5- and 2,6-lutidine, collidine, pyrazine, substituted triazines, Pyrimidines and fused cyclic derivatives such as quinoline, isoquinoline, benzimidazole and substituted benzimidazoles such as 2-aminobesimidazole. Examples of ineffective or less effective compounds in which the nitrogen atom has a steric hindrance are 2,6-diphenylpyridine, 2,6-t-butylpyridine and pyridine-N-oxide.
Similarly, the coordinating heteroatom should not be protonated; In the case of a nitrogen-containing heterocyclic compound, the pH of the leaching system must be greater than pK a of the protonated nitrogen atom of the compound. The sulfur-containing compounds according to the invention and should not have steric hindrances. Examples of suitable sulfur-containing compounds are thiophene, 3-thiophenecarboxylic acid and 3-thiophenacetonitrile. Ineffective or less effective thiophene derivatives include tetrabromothiophene and 3,6, 9,14-tetrathyabicyclo [9.2.1] tetradeca-11, 13-diene. Inactivity of the compounds does not necessarily imply a lack of binding to the metal surface.
By heterocyclic aromatic compounds Applicants mean compounds falling within the conventional definition of "aromatic" widely used in organic chemistry (J.Marh, Advanced Organic Chemistry, 4th ed., Wiley & Sons, 1992). These include organic cyclic compounds containing at least one heteroatom (or non-carbon atom) in which the ring is closed or a fused, fully conjugated system with 4n + 2 (n = 0,1,2,3 .. .) By pi-electrons in the conjugated system. For example, pyridine, a 6-membered ring with a nitrogen atom, is such a heterocyclic aromatic compound in which nitrogen and each carbon atom in the ring give off one electron per pi system of common 6 pi electrons. Thiophene, a 5-membered heterocycle with a sulfur atom, and is aromatic, with a sulfur atom giving up two electrons, and each of the carbon atoms giving up one electron to a common system of 6 pi-electrons. Aromatic character and suggests other important features of the molecules used in this invention. The cycles described above have a planar or planar structure with a heterocyclic atom as part of the planar structure of the molecule. However, it is not necessary that the molecule of the active compound be completely planar. Substituents can significantly affect the activity of heterocyclic compounds by altering the aromaticity of the ring. Both 2-hydroxypyridine and 4-hydroxypyridine are ineffective as gold cyanidation catalysts, whereas 3-hydroxypyridine is an effective catalyst. Inertness is the result of the fact that 2- and 4-hydroxy derivatives are present in the form of ketotautomers, which, thus, attenuates the aromatic character and promotes the protonation of pyridine nitrogen. This tautomer does not matter for 3-hydroxypyridine, which remains completely aromatic with a non-protonated nitrogen atom. Similar examples can be given for other nitrogen-containing aromatic ring systems.
A variety of substituents on the aromatic heterocyclic ring may be tolerant or even enhance the activity of the parent heterocyclic aromatic compounds. The starting compounds include, for example, pyridine, pyrazine, pyrimidine, imidazole, oxazole, pyrazole, thiazole, thiadiazole, thiadiazole, thiazine, thiophene and triazole, as well as condensed heterocyclic derivatives such as quinoline, isoquinoline, benzimidazole, benzothiophene. Substituents include alkyl groups (such as methyl, ethyl, propyl and butyl), alkoxy groups (such as methoxy and ethoxy), aryl groups (such as phenyl and tolyl), heterocyclic aromatic groups (such as pyridyl, pyrazolyl, imidazolyl and thiophenyl ), Amino and substituted amino groups (such as in 4-dimethylaminopyridine), carboxy, acetyl and halide (fluoride, chloride, bromide, iodide). A combination of substitutes and effective; For example, amino and hydroxyl substituents on the alkyl chain as a pyridine substituent (eg 2- (2-hydroxyethyl) pyridine) give the active compounds. Nitrogen-containing heterocyclic aromatic compounds with substituents that increase the pK a of the nitrogen atom in the ring are more effective activators allowing the leaching system to operate at a suitable pH range.
The compounds can be tested for their action as activators electrochemically. To compare solutions of interesting ligands that contain and do not contain the putative activator, cyclic voltammograms are used. In this method, the voltage is changed between two electrodes (a working electrode made of gold and an indicator electrode, such as platinum), by measuring the current between the electrodes. If an oxidation or reduction reaction takes place on the electrode at a certain voltage, a current flows between the electrodes. When the gold electrode dissolves, the current increases for a given voltage. This electric current is called the oxidation wave or the anode peak, the potential at which this occurs is the oxidation potential. Without an activator or with an inefficient connection, the oxidation wave is observed only at the oxidation potential determined by the ligand in the solution. In the presence of an activator, an additional wave is observed in the passive section of the voltammogram of the non-activated variant. This new wave is shifted toward the cathode (at a lower potential) from the peak in the inactive version.
For example, in a chloride solution, the process giving an anode peak is as follows:
Au + 4Cl - ---> [AuCl 4 ] - +3 electrons,
This process corresponds to the anodic dissolution of gold. In the presence of even low concentrations of the activator, an additional peak is observed, closer to the cathode than the first peak, but the same gold compound is obtained. That is, the activator compound activates the dissolution of the gold electrode.
It is important that a new oxidation wave occurs as a result of the combination of a ligand (such as a chlorine ion) with an activator (such as pyridine) in a synergistic manner, which actually shifts the oxidation wave of gold to a lower potential. Similar effects with respect to gold oxidation potentials can be observed with various combinations of activator (such as pyridine, imidazole, N-methylimidazole or 3-thiolphenecarboxylic acid) and a ligand (such as chloride, bromide, iodide, thiocyanate, malononitrile or cyanide). In some cases, such as with a thiosulfate as a ligand, a new peak is not observed, rather the current associated with the oxidation of gold increases significantly with the addition of low concentrations of an activator such as pyridine. An increase in the electric current indicates an increase in the anodic dissolution of gold in the thiosulfate solution.
This new or enhanced peak is not observed in solutions of the activator with the anion, which does not form a coordination bond with gold (such as perchlorate or nitrate); Thus, the new peak is not the only result of the interaction of the activator with the gold surface.
Cyclic voltammograms of the gold electrode in the solutions of the activator are very different. Indeed, for most nitrogen-containing heterocyclic compounds under non-acidic conditions, there is no data on the dissolution of the gold electrode.
The previous experience does not contain data on this possibility of activators to shift the oxidation wave to a lower potential. Indeed, when pyridine is used in solutions containing anions such as perchlorate that does not form a stable gold complex, it has been found that pyridine increases the potential of the oxidation wave and therefore protects the gold surface from anodic reactions (P. Zelenay, LM Rice-Jackson And A. Wieckowski, Langmuir, 6, 974-979 (1990)).
Since there is no complete list of possible nitrogen and sulfur-containing aromatic heterocycles according to the invention, the following list shows the most preferred compounds: 2- (aminomethyl) pyridine, 2-aminopyridine, 3-aminopyridine, 4-aminopyridine, 2,2'-dipyridyl , 2-propylpyridine, 2,2'-pyridyl, pyridine, 3-aminopyrazole, 3,5-dimethylpyrazole, 4,4'-dipyridyl, 2,2'-dipyridylamine, di-2-pyridylketone, 4-t-butylpyridine, 2-chloropyridine, 2,4,6-collidine, 4-dimethylaminopyridine, 2-ethylpyridine, 2-fluoropyridine, 2- (2-hydroxyethyl) pyridine, 3-hydroxypyridine, 2,3-lutidine, 2,4-lutidine, 2 , 6-lutidine, 3,5-lutidine, 2-methoxypyridine, nicotinamide, nicotinic acid, 2-phenylpyridine, 3-phenylpyridine, 4-phenylpyridine, 2-picoline, 3-picoline, 4-picoline, 2-thiophenecarboxylic acid, 3 -thiophenecarboxylic acid, 4-methylpyrazole, 3-methylpyrazole, 1-phenylpyrazole, pyrazole, 1- (3-aminopropyl) imidazole, 2,2'-bis (4,5-dimethylimidazole), 4,5-dicyanoimidazole, 1,2 Dimethylimidazole, 2-ethylimidazole, histamine, histidine ethyl ester, imidazole, N-methylimidazole, 2-methylimidazole, 4-methylimidazole, N-phenylimidazole, 2-acetylthiophene, 3-bromothiophene, 2,5-dimethylthiophene, 2-methylthiophene, 3 Methylthiophene, thiophene, 3-thiopheneacetic acid, quinoline, 2-aminobenzimidazole,
2-thiophenemethanol, 2-aminothiazole, 3-amino-5,6-dimethyl-1,2,4-triazine, 2-amino-1,3,4-thiadiazole, 2,4,5-trimethyloxazole, trimethylpyrazine, benzimidazole, Benzothiazole, benzotriazole, isoquinoline, 1,10-phenanthroline, 2-aminoimidazole, aminopyrazine, 2-aminopyrimidine, 2-amino-4,6-dimethylpyrimidine, 2,6-diaminopyridine, L-histidine, DL-histidine, pyrazine, tetramethylpyrazine, Trimethylpyrazine.
LIGAND / OXIDATOR SYSTEMS
The leaching systems of the invention are those systems generally known in the art that are effective in dissolving gold using a ligand / oxidant system, and in particular including cyanide ion and air or dissolved oxygen; Chloride ion with sodium hypochlorite (a stabilizer, such as sulfamic acid, can be used); Bromide ion with bromine, in the presence of a stabilizer, such as sulfamic acid; Iodide and iodine; Thiocyanate and ferric ions; Thiourea and ferric ions; Thiosulfate anion and copper ion as a catalyst in the presence of ammonia and air or dissolved oxygen; Malononitrile and air or dissolved oxygen and cyanide, chloride, bromide, iodide, thiocyanate, thiosulfate, molononitrile or thiourea and a gold electrode with a potential corresponding to each of these ions. Each of the activators listed above may not be suitable for all leach solutions due to adverse reactions, pH, or poor binding to the gold surface under reaction conditions in a particular system.
The choice of ligand concentration, oxidant concentration, activator concentration and pH depends on the system to be used for extraction. The appropriate extractant activator combinations can be checked electrochemically, as described below.
Cyanide with air or dissolved oxygen is the preferred leaching system most widely used for gold recovery (for example, gold mining and gold extraction in electroplating). EBSaubestre in "Modern Electroplating", EA Lowenheim, Ed., John Wiley and Sons, Inc., New York, 1953, p. 748-770. Such systems typically operate at alkaline pH values due to the formation of HCN, which lowers the pH below 10 (pK a HCN 9.1). The choice of the concentration of cyanide and activator depends on the specific application. When measuring the dissolution rate of gold as gold powder, foil, bars or discs with air, the best results are obtained by low concentrations of cyanide (2-10 mM cyanide). However, electrochemical studies show that activation of gold dissolution can be observed over a wide range of cyanide concentrations. When used in the gold mining industry, the choice of cyanide concentration depends on many factors. A low concentration of activators (1-10 ppm) is preferred at standard concentrations of cyanide. Alkaline pH values, which are usual for cyanidation reactions, are suitable for activators. In some cases, cyanidation is carried out at a lower pH (<9). The activator under these conditions must be chosen so that the heteroatom in the heterocyclic ring is not protonated. A wide range of activators is suitable for cyanide systems.
Preferred compounds are imidazole, N-methylimidazole, 1,2-dimethylimidazole, pyridine, alpha, beta, gamma-picoline, 2-, 3-, 4-aminopyridine, 4-dimethylaminopyridine, thiophene, 3-thiophenecarboxylic acid, 3- Thiopheneacetic acid, pyrazine, 2,4,6-trimethyltriazine, thiazole, L-histidine, 2-aminopyrimidine, nicotinamide, 2-amino-4,6-dimethylpyrimidine. Most preferred are imidazole, N-methylimidazole, L-histidine and 2-aminopyrimidine.
The chloride-hypochlorite system can be used as an alternative to cyanide. See, for example, JB Hiske and VP Atluri, Mineral Processing and Extraction Metallurgy Review, 4.95-134 (1998), and US Patent No. 5.169.503, incorporated herein by reference. This system usually works at more than 3% NaCl and pH 5-8. The use of activators makes it possible to operate the system under less corrosive conditions with a lower chloride concentration. The preferred chloride leach system with activators consists of 2-5% NaCl (or other chloride source), 0.1% sodium hypochlorite with a pH of 8-8.5. In both cases, a hypochlorite stabilizer is required. Stabilizers are compounds in which chlorine is still active, but is in a more stable form. The stabilizer must be chosen such that it does not participate in the gold dissolution reaction. A suitable stabilizer for the sodium chloride-sodium hypochlorite leach solution is sulfamic acid. In this case, sulfamic acid reacts with hypochlorite anion to form N-chlorosulfamic acids, in which the chlorine is still active. See, for example, "Kirk-Othmer Encyclopedia of Chemical Technology," v. 21, John Wiley and Sons, New York, 1983, p.949-956.
The activator should be selected so that the heteroatom of the heterocyclic aromatic compound is available to form a coordination bond with the gold surface and no interaction with other components of the leach solution occurs. Preferred activators are pyridine, alpha, beta, gamma-picoline, di-2-pyridyl ketone, 2,2-dipyridyl, 3,5-lutidine, 2,6-lutidine, 2,3-lutidine and N-methylimidazole.
The bromide-bromine system can be used as an alternative to cyanide. See, for example, JBHiskey and VP Atluri, Mineral Processing and Extractive Metallurgy Review, 4.95-134 (1988); J.Marsden and I.House, "The Chemistry of Gold Extraction", Ellis Horwood, New York, 1992, pp.304-305; And A. Dadger, Journal of Minerals, Metals and Materials Society, 41, 37-41 (1989), which are incorporated by reference. The arguments for choosing the activator are the same as for the chloride-hypochlorite system. The preferred bromide system containing the activator consists of 1% NaBr (or other bromide ion source), 0.3% bromine in the presence of sulfamic acid, pH 9.0. Preferred activators are pyridine, alpha, beta, gamma-picoline, di-2-pyridylketone, 2,2'-dipyridyl, 3,5-lutidine, 2,6-lutidine, 2,3-lutidine, 2,3-lutidine and N-methylimidazole.
The thiosulfate-copper-ammonia-air system or dissolved oxygen can be used as an alternative to cyanide. See, for example, JB Hiskey and VP Atluri, Mineral Processing and Extractive Metallurgy Review, 4.95-134 (1988); J. Marsden and I. House, "The Chemistry of Gold Extraction," Ellis Horwood, New York, 1992, pp. 303-304; C.Changlin, H.Jiexue and G.Qian, "Leaching Gold by Low Concentration Nhisulfate Solution", Randol Gold Forum, Vancoucer '92, March 25027, 1992, pp. 293-298, J.Tao, C.Jin and X.Shi in "Hydrometallurgy", JB Hiske and GWWarren, Eds., Society for Mining, Metallurgy and Exploration, Jnc. , Littleton, CO, 1993, pp. 119-126; And MJ Nicol, CA Fleming and RL Paul in "The Extractive Metallurgy of Gold in South Africa", Volume 2, GG Stanley, Ed., South African Institution of Mining Metallurgy, Johannesburg, 1987, 831-905, which are incorporated herein in the form of Links. In the thiosulfate system, the following range of reagent concentrations can be used: thiosulfate ion-0.1-0.25 M; The copper (II) ion is 0.01-0.15 M; ион аммония (в виде тиосульфата, гидроксида и сульфата) - 0,5 - 4,8 М. Предпочтительными условиями являются 0,1 М тиосульфат натрия, 0,01 М сульфат меди и 0,5 М гидроксид аммония. Предпочтительными активаторами являются пиридин, альфа-, бета-, гамма-пиколин, ди-2-пиридилкетон, 2,2'-дипиридил, 3,5-лутидин, 2,6-лутидин, 2,3-лутидин, имидазол, N-метилимидазол, тиофен, 3-тиофенкарбоновая кислота, 3-тиофенуксусная кислота, пиразин, 2,4,6-триметилтриазин и тиазол.
Другие выщелачиватели могут использоваться аналогично, то есть, к системе при обычных условиях, применяемых в данной выщелачивающей системе, добавляется соответствующий активатор. Систему йодид-йод применяют в качестве выщелачивания золота. Для использования подходит широкий диапазон концентраций в широком интервале pH. Однако приемлемую скорость растворения золота дает 0,03 М йодид натрия в присутствии 0,005 М йода при pH 5. (PHQiand, JB Hiske "Hydrometallurgy", 27, 47-66 (1991) и FW Devries and JBHiskey, "Environmental Impact of Lixiviants: An Overview That Includes Noncyanide Chemistry", Randol Gold Forum, Vancouver'92, March 25-27, 1992, p.89-92). Псевдогалогенид тиоцианат и используют в качестве выщелачивателя золота. J. Marsden and I.House, "The Chemistry of Gold Extraction", Ellis Horwood, New York, 1992, p.303-304; и O.Barbosa and AJMonhemius in "Precious Metals'89", MC Jha and SDHill, Eds., The Minerals, Metals amd Materials Society, p. 307-339 (1988)). Реакционная среда с этим лигандом обычно содержит 10-15 г/л тиоцианата натрия и 1-10 г/л иона трехвалентного железа в качестве окислителя в интервале pH 2-3. Малононитрил - другой псевдогалогенид, который используется в качестве выщелачивателя золота в присутствии воздуха или кислорода. (HJHeinen, JAEisele and BJScheiner, "Malononitrile Extraction of Gold From Ores", Bureau of Mines Report of Investigation, 7464, 1970 и
KRSandgren and JE Murphy in "Hydrometallurgy", JB Hiskey and GW Warren, Eds., Society For Mining, Metallurgy and Exploration, Inc., Littleton, CO, 1993, p. 301-310). В реакции с этим лигандом обычно используют 0,01-1,0% малононитрил в широком интервале pH (7,5-12). Тиомочевина - еще одно соединение, которое применяется как выщелачиватель золота с ионами трехвалентного железа или пероксидом водорода в качестве окислителя (J. Marsden and I.House,"The Chemistry of Gold Extraction", Ellis Horwood, New York, 1992, p.299-302; MJNicol, CAFleming and RL Paul in "The Extractive Metallurgy of Gold in South Africa", V. 2, GGStanley, Ed., South African Institution of Mining Metallurgy, Johannesburg, 1987, p.845-846; JBHiskey and VPAtluri. Mineral Processing and Extractive Metallurgy Review, 4,95-134 (1988). В реакции с этим лигандом используется обычно тиомочевина (1-5 г/л) и ион трехвалентного железа (0,5-5 г/л) при pH 1-3.
Ароматические гетероциклические соединения изобретения и реагенты, используемые в описанных выщелачивающих системах лиганд/окислитель, хорошо известны в этой области и продаются. Изобретение проиллюстрировано следующими неограничивающими его примерами.
Example 1 . Activation of gold dissolution in sodium chloride solution - hypochlorite at pH 8.5
It is shown that at this high pH value, sodium hypochlorite itself is a very weak oxidant in order to induce any significant dissolution of gold. However, in the presence of an activator, this weak oxidant becomes very effective.
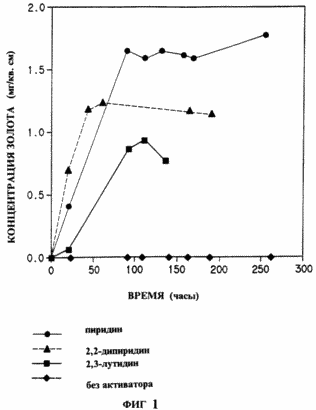 |
In this example, the addition of activator compounds allows hypochlorite to be used as an oxidizer under conditions in which, in other cases, gold dissolution is negligible or completely absent. The reactions were carried out in a buffer solution pH 8.5, prepared as follows. Sodium borate (0.955 g) and sulfamic acid (0.05 g) were mixed with about 40 ml of HPLC purified water (pure for high performance liquid chromatography). The mixture was stirred until completely dissolved. Sodium chloride (1.25 g) was added. The pH of the solution was adjusted to 8.5 1 N HCl. Standardized sodium hypochlorite was added to 0.1% by weight of the solution and the pH was again adjusted to 8.5. The solution was then transferred to a 50 ml volumetric flask. An activator was added to the solution. The volume of the solution was adjusted with HPLC water of purity. An aliquot of 2 ml was taken at time 0. The remaining solution was poured into a vessel with a previously measured piece of gold foil (1 cm x 5 cm x 0.1 mm) and periodically aliquots were taken to analyze the gold content. Samples were analyzed by converting the dissolved gold complex into [AuBr 4 ] - by extracting the bromide into chloroform with trioxylphosphine oxide and measuring the absorbance of this solution to determine the gold concentration (FFBeamish ahd JC Van Loon, "Recent Advances in the Analytical Chemistry of The Noble Metals" Pergamon Press, New York, 1972, p.322-323 and WNNolbrook and E. Rein, Analytical Chemistry, 36, 2451-2453 (1964) Gold dissolution rates were calculated by standing the graph of the amount of dissolved gold (microgram of gold per sq. Cm. Foil) relative to the time in hours.The number of hours was fixed at the point where the concentration of gold reached a maximum.In the table below, the activators used and the results obtained are listed: |
In Fig. 1 shows graphically typical measurements of the concentration of dissolved gold versus time for several reactions.
Example 2 . Activation of gold dissolution in sodium bromide-bromide solution with pyridine.
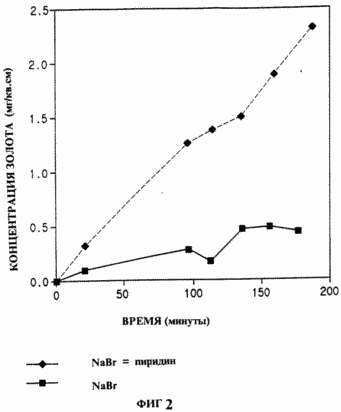 |
In this example, the activator catalyzes the dissolution of gold in a solution containing sodium bromide and an oxidant obtained by adding bromine to the basic solution; With the formation of hypobromide (RCTroy and DW Margerum, Inorganic Chemistry, 30, 3538-3543 (1991) .Sodium bromide (1.00 g), sodium borate (1.910 g) and sulfamic acid (0.10 g) were mixed with 90 ml Water of HPLC purity.The pH of the solution was adjusted to NaOH to 9.0 The mixture was stirred in ice 0.25 hours 100 μl of bromine was added The mixture was stirred at ice temperature 0.5 hours and then warmed to room temperature The pH of the solution was adjusted to 9 with NaOH, 0, the solution was transferred to a 100 ml volumetric flask and brought to full volume.The solution was then divided into 2 parts: 50 ml was mixed with a gold foil sample of the same dimensions as in Example 1, the other 50 ml was mixed with 100 μl of pyridine and mixed with Another sample of gold foil. At intervals, aliquots were taken and analyzed as in Example 1. The results are shown graphically in FIG. 2. The sample with pyridine clearly shows a higher dissolution rate of gold than the sample without pyridine. |
Example 3 . Activation of gold dissolution in sodium cyanide solution by a nitrogen-containing heterocycle
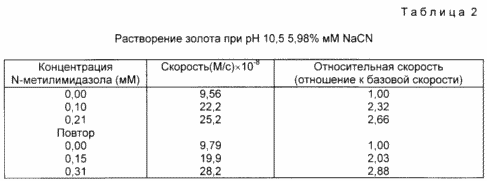
In this example, the addition of an activator allows the rate of dissolution of gold in the sodium cyanide-air system to be increased. The reaction was carried out in a solution of NaCN at pH 10.5, prepared as follows. NaCN (0.033 g) was added to 100 ml of water and 10 ml of 0.10 M potassium phosphate buffer at pH 11. The final pH of the solution was adjusted to 10.5 by the addition of K 2 HPO 4 . The cyanide concentration was determined so that it was 5.98 mM, titrated with silver nitrate. A gold sample (area 0.495 sq. Cm.) Was obtained by vacuum deposition of a 2000 A gold layer onto a plastic substrate. A solution of sodium cyanide (2.50 ml) was added to the gold sample, and UV control was set to 0 against the stock solution. The solution was stirred at 25 ° C. and the absorbance (at 240 nm) of the formed [AU (CN 2 ) - every 5 seconds for 200 s was measured.) A zero-order rate constant was obtained by conducting a better line through the points obtained. After 200 seconds, 20 μl of 0.013 M solution of N-methylimidazole, previously prepared by the addition of 0.10 g of N-methylimidazole to 100 ml of water.After 400 s were observed for the formation of [Au (CH) 2 ] , and the rate constant of zero order was determined as previously described. In the presence of an activator, was obtained by dividing the zero-order rate constant with the activator by a rate constant without an activator.The results, including a retest, are presented in Table 2.
Example 4 . Activation of gold dissolution in a sodium cyanide solution with a sulfur-containing heterocycle
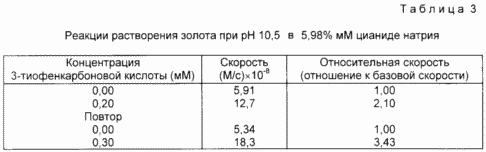
In this example, the addition of an activator makes it possible to increase the dissolution rate of gold in the cyanide solution. The reactions were carried out in buffered NaCN solutions at pH 10.5 prepared as follows. NaCN (0.027 g) was added to 100 ml of water and 10.0 ml of 0.10 M phosphate buffer at pH 11. The pH of the solution was adjusted to 10.5 by the addition of K 2 HPO 4 . The cyanide concentration was determined (it should be 4.74 mM) by titration of silver nitrate. A sample of gold (surface area 0.495 cm2) was prepared as in Example 3. A solution of sodium cyanide (2.00 ml) was added to the gold sample and UV control of this solution was carried out. The solution was stirred and kept at 25 ° C. during the dissolution reaction. The absorbance of the samples at 240 nm associated with the formation of [Au (CN) 2 ] during the reaction was measured every 5 seconds for 100 s and a zero-order rate constant was obtained by conducting a better straight line through the obtained points. A 9.67 mM solution of 3-thiophenecarboxylic acid was prepared by adding 0.031 g to 25 ml of a solution containing 60 parts (by volume) of water and 40 parts of methanol. Then, 64 μl of a solution of 3-thiocarboxylic acid was added to the gold sample in a buffered cyanide solution, as described above. The formation of [Au (CN) 2 ] was followed by another 100 s and the rate constant of zero order was determined as described earlier. The relative dissolution rate of gold in the presence of the activator was obtained by dividing the zero-order rate constant in the presence of the activator by the rate constant without the activator. The results, including repeated tests, are presented in Table. 3.
Example 5 . Activation of gold dissolution in a solution of sodium thiosulfate with pyridine.
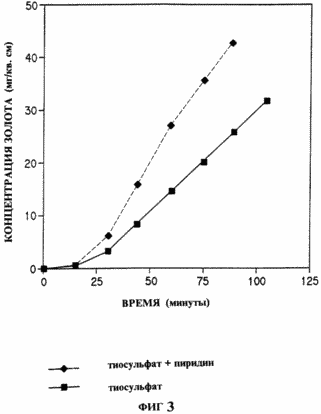 |
In this example, the addition of an activator catalyzes the dissolution of the gold foil in an alkaline thiosulfate solution with a copper ion added as an oxidation catalyst. The thiosulfate solution was prepared by dissolving sodium thiosulfate (1.242 g Na 2 S 2 O 3 .5 H 2 O) and copper sulfate (0.079 g) in about 40 ml HPLC water of purity. Ammonium hydroxide (5 ml of concentrated NH 4 OH) was then added. The volume of the solution was adjusted in a volumetric flask to 50 ml, obtaining 0.1 M sodium thiosulfate, 0.01 M copper sulfate and 0.5 M ammonium hydroxide. At time 0, the concentration of gold was determined by taking 0.5 ml of the solution, adding 1.0 ml of royal vodka in a 10 ml volumetric flask and bringing the volume up to the mark. The gold concentration was determined by atomic absorption spectroscopy in a graphite furnace. The remaining solution was transferred to a screw-capped vessel. A piece of gold foil (1 cm x 5 cm x 0.1 mm) was added and the vessel was closed. Aliquots (0.5 ml) were withdrawn every 15 min for gold analysis as described above. In Fig. 3 shows the concentration of gold in this study. The procedure described above was repeated in the presence of a pyridine activator. After transferring the ammonium copper-thiosulphate solution to a screw-capped vessel, 1 μl of pyridine and then a piece of foil were added. Gold concentration analysis was carried out as described above. A comparison of the two time curves in FIG. 3 shows that in the pyridine example, the dissolution rate is much higher than without the activator. |
Example 6 . Activation of the dissolution of gold from oxidized gold ore in a solution of sodium cyanide with a nitrogen-containing heterocycle.
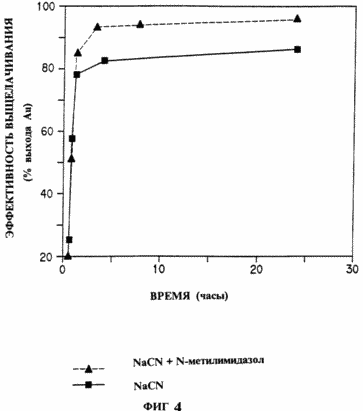 |
This experiment was conducted with gold ore, which was crushed and crushed to such an extent that 80% passed through a 200 mesh sieve. The ore was mixed with water to form a slurry of 40-50% solids concentration. Sodium cyanide was added until a solution of a final concentration of 0.4 g NaCN / liter was obtained. The gold concentration was determined by atomic absorption spectroscopy. In Fig. 4 shows the time curves of the ore-miscible leach solution described above and the above solution with 5 mg / L of added N-methylimidazole. In Fig. 4 that N-methylimidazole catalyzes the dissolution of gold in cyanide solutions. The catalyzed reaction reaches a maximum of the leaching efficiency of gold much faster than the non-catalyzed reaction. |
Example 7 . Effect of nitrogen-containing activators on gold electrodes in the presence of various ligands.
This example demonstrates the effect of the activators of pyridine and N-methylimidazole on the cyclic voltammogram of the gold electrode in the ligand solution. All potentials are given relative to the silver-chloride chloride reference electrode. Ligands are chloride, bromide, iodide, thiocyanate, thiosulfate and malononitrile. Gold electrodes for each ligand solution were prepared by galvanic deposition of a gold layer on a glassy carbon electrode from a solution of gold chloride in HPCL water of purity, first conducting oxidation of the electrode at 1.00 V to a current of 0.01 mA or less and then applying gold from an unmixable solution [ Au Cl 4 ] - in a nitrogen atmosphere at 0.00 and 5-10 minutes. The conditions for obtaining the voltammogram are shown in the table below. 4. Unless otherwise specified, the electrolyte in all cases is 0.05 M KF. A cyclic voltammogram was first recorded for each ligand with a solution without an activator. Then, the activator was added to the desired concentration and a cyclic voltammogram was recorded in the same range of potentials.
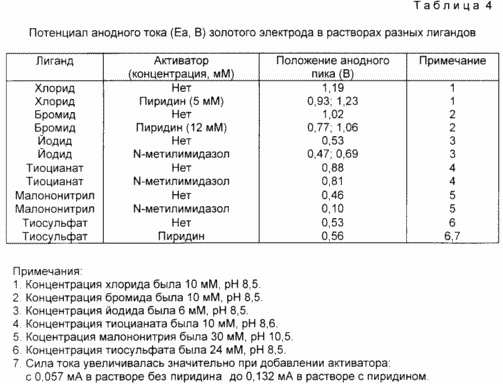
Two types of responses were observed, each corresponding to the activation of the gold dissolution reaction. With halides and pseudohalides (chloride, bromide, iodide, thiocyanate, malononitrile and cyanide), a new oxidation wave (anodic peak) appears at a lower potential. In some cases, such as cyanate, a new wave overlaps with a dissolution wave in a ligand solution without an activator.
With the thiosulfate anion, a new wave was not observed, moreover, when the activator (pyridine) was added to the electrolyte solution, a significant increase in the current was observed at the oxidation potential Au (0) / Au (1). This example clearly showed that pyridine catalyzes the dissolution of gold in a solution of thiosulfate.
In conclusion, the two answers shown in this table are the appearance of new waves with a lower potential, cathodic with respect to the Au (0) / Au (1) pair, in the ligand solution and / or a significant increase in current at this potential. The type of response depends on the type of ligand used in the study. Each of the answers indicates an increase in the dissolution rate of gold and, consequently, activation of the leaching system.
Example 8 . The effect of heterocyclic aromatic compounds on gold electrodes in a solution of cyanide
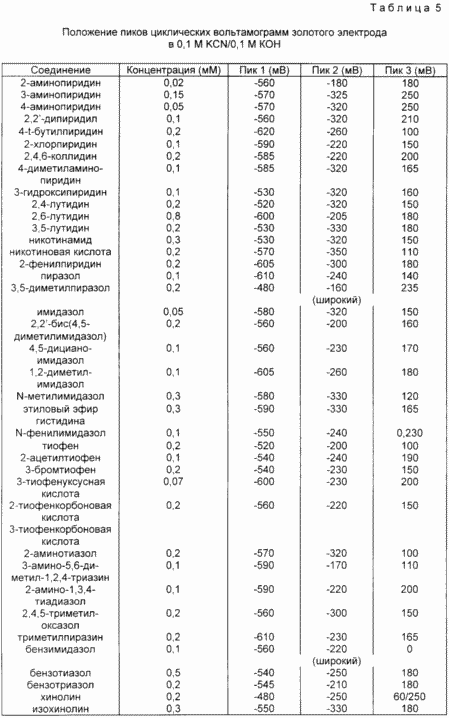
In this example, the effect of various heterocyclic aromatic compounds on the cyclic voltammetry of a gold electrode in a solution of 0.1 M KCN / 0.1 M KOH was studied. The gold electrode served as a selling electrode, which was grinded with 6 micron diamond paste before use, washed with deionized water and acetone, soaked in 30% hydrogen peroxide, and washed with deionized water. Before placing the electrode in a KCN solution, this solution was carefully de-aerated with nitrogen. Cyclic voltammograms were obtained on a voltammograph BAS CV27 (Bioanalytical Systems, Inc., 2701 Kent Avenue, West Lafayette, IN 47906) using a platinum wire electrode and an Ag / AgCl reference electrode. The gold electrode was tested by applying a voltage (-800 mV to the Ag / AgCl electrode) for 2 minutes, stirring the solution with a magnetic stirrer. A voltammogram was then obtained for an immiscible solution protected by nitrogen. The electrode test was repeated. At this point, an aromatic heterocyclic compound was added. The solution was stirred for 2 min. Without imposing a voltage, for weaker activators, a longer incubation period was required. Then a cyclic voltammogram of the solution was recorded.
In Table. 5 shows the waves of KCN / KOH solutions with various organic compounds. The activators are characterized by a wave in the region from -180 to -350 mV (peak 2), which is absent in the KCN / KOH solution without the activator. In some cases, an additional wave is observed at lower potentials (ca. -600 mV). Non-activated KCN / KOH solutions show a wave in the + 200 mV region.
Example 9 . Effect of a sulfur-containing heterocyclic activator on the gold electrode in a solution of thiourea.
In this example, the effect of the activator of 3-thiophenecarboxylic acid on the cyclic voltammogram of the gold electrode in an acidic solution of thiourea is shown. The electrochemical equipment described in Example 8 was used. To prepare solutions and wash chemical dishes and electrodes, water from Barnstead Nanopure System (Barnstead Thermolyne Corporation, 2555 Kerper Boulevard, Dubuque, IA 52001, USA) was used. The working electrode was a gold voltammetric electrode, a silver electrode, and an electrode made of platinum wire. They were used as reference electrodes and counting electrodes; All the indicated potentials are given with respect to the silver electrode. All electrodes were obtained from (Bioanalytical Systems, Inc., 2701 Kent Avenue, West Lafayette, IN 47907, USA).
A solution of 0.10 M sodium sulfate was prepared by dissolving 0.710 g of anhydrous sodium sulfate in about 40 ml of water in a 50 ml beaker. The pH of the solution was adjusted with concentrated sulfuric acid to 2.5. The solution was transferred to a 50 ml volumetric flask and the volume was adjusted to a mark. This solution was used as a supporting electrolyte in the preparation of a cyclic voltammogram. A cyclic voltammogram was obtained in the potential range from + 0.400V to - 0.400 V; The sweep speed in this example was 50 mV / s. The initial potential was -0.20 V; The superimposed potential was deployed in the direction of the anode to 0.40 V; At this point the scan was rotated in the opposite direction to -0.400 V. No waves were observed.
To 10 ml of the supporting electrolyte solution in an electrochemical cell, 0.076 g of thiourea was added until a 0.10 M solution was obtained. A cyclic voltammogram was obtained in the interval described above. The oxidative wave was observed at approximately +0.335 V; In the reverse direction of the sweep, the recovery wave was observed at 0.00 V. These waves correspond to the oxidation of gold with the formation of a gold-thiourea complex and to the reduction of the gold-thiourea complex with the formation of metallic gold, respectively.
A 0.2 M solution of 3-thiophenecarboxylic acid was prepared by dissolving 0.380 g in 25 ml of absolute ethanol.
To a solution of thiourea in an electrochemical cell, 2 μl of the above-described solution of 3-thiophenecarboxylic acid in ethanol was added. The solution was stirred for about 30 minutes. A cyclic voltammogram was prepared as described above. The current, both for the oxidation wave and for the reduction wave, increased, indicating enhanced gold dissolution and recovery of the gold-thiourea complex, respectively. Additional aliquots of a solution of 3-thiophenecarboxylic acid in ethanol were added until the total volume of the additives was 4, 6 and 8 ml; The solution concentrations were thus in the range of 0.04-0.16 mM. After each addition, the solution was stirred for 15-30 minutes. A cyclic voltammogram was then obtained in the above potential interval. The current of the oxidation and reduction waves increased with an increase in the concentration of the activator from 0.04 to 0.16 mM, indicating an increase in the dissolution of gold in the activator solution.
Example 10
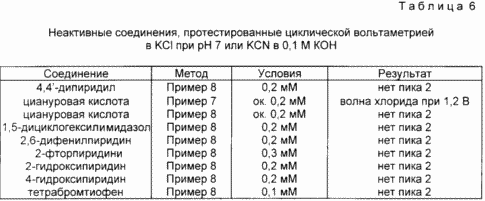
Comparative examples of inactive nitrogen- and sulfur-containing heterocyclic aromatic compounds
In this example, some nitrogen- and sulfur-containing heterocyclic aromatic compounds are shown which do not activate the gold dissolution reaction using the methods described above. In Table. 6 lists inactive compounds, as well as the methods used to verify activity, and the results obtained. The cyclic voltammetry of Example 7 was used to demonstrate the appearance of a new oxidizing wave cathodically displaced from the wave observed in a solution of one ligand. KCl was used as the ligand (all solutions were prepared as described above). No new wave was observed, indicating that the connection was inactive. In Example 8, a solution of 0.1 M KCN / 0.1 M KOH was used to demonstrate the activation of gold dissolution in the cyanide solution. Active compounds give a new wave (Peak 2), cathodically displaced from the peak observed in the ligand solution. The absence of a new wave indicates the lack of activation of the gold dissolution reaction.
CLAIM
1. A method for dissolving gold in a ligand / oxidizer leaching system, comprising adding a catalytic amount of a reagent to the system, characterized in that an aromatic heterocyclic compound containing nitrogen and / or sulfur in the ring is used as the reagent, provided that the sulfur and / or nitrogen heteroatom And a heterocyclic ring are available to form a coordination bond with the surface of undissolved metallic gold.
2. The process of claim 1, wherein the ligand / oxidant leaching system is a cyanide ion with air or dissolved oxygen.
3. The method of claim 1, wherein the ligand / oxidant leachate system is a chloride ion with sodium hypochlorite at a pH of 8 to 9, optionally in the presence of a stabilizer.
4. The process of claim 1, wherein the ligand / oxidant leaching system is a bromide ion with bromine, optionally in the presence of a stabilizer.
5. The method of claim 1, wherein the ligand / oxidant leaching system is iodide ion with iodine.
6. The process of claim 1, wherein the ligand / oxidant leaching system is thiocyanite with ferric ions.
7. The process of claim 1, wherein the ligand / oxidant leaching system is thiourea with ferric ions.
8. The method of claim 1, wherein the ligand / oxidant leaching system is a thiosulfate anion with copper, ammonia and air or dissolved oxygen.
9. The method of claim 1, wherein the ligand / oxidant leaching system is malononitrile with air or dissolved oxygen.
10. The method of claim 1, wherein the ligand / oxidant leaching system is cyanide, chloride, bromide, iodide, thiocyanate, thiosulphate, malononitrile or thiourea in the presence of a charged gold electrode.
11. The method of claim 1, wherein the catalytic compound is an aromatic heterocycle with a nitrogen and / or sulfur atom in an aromatic ring which, when tested in a solution of 0.1 M KCN / 0.1 M KOH containing a gold electrode, shows a voltametric wave in Range from -180 to -350 mV.
12. The method of claim 1, wherein the catalytic compound is selected from the group consisting of 2- (aminomethyl) pyridine, 2-aminopyridine, 3-aminopyridine, 4-aminopyridine, 2,2-dipyridyl, 4,4-dipyridyl, 2, 2-dipyridylamine, di-2-pyridylketone, 4-butylpyridine, 2-chloropyridine, 4-phenylpyridine, 2-picoline, 3-picoline, 4-picoline, 2-propylpyridine, 2,2'-pyridyl, pyridine, 3-aminopyrazole , 3,5-dimethylpyrazole, 4-methylpyrazole, 2,4,6-collidine, 4-dimethylaminopyridine, 2-ethylpyridine, 2-fluoropyridine, 2- (2-hydroxyethyl) pyridine, 3-hydroxypyridine, 2,3-lutidine, 2,4-lutidine, 2,6-lutidine, 3,5-lutidine, 2-methoxypyridine, nicotinamide, nicotinic acid, 2-phenylpyridine, 3-phenylpyridine, N-phenylimidazole, 2-acetylthiophene, 3-bromothiophene, 2.5 -dimethylthiophene, 2-methylthiophene, 3-methylthiophene, thiophene, 3-thiopheneacetic acid, 2-thiophenecarboxylic acid, 3-thiophenecarboxylic acid, 2-thiophenemethanol, 2-aminothiazole, 3-amino-5,6-dimethyl-1,2, 4-triazine, 3-methylpyrazole, 1-phenylpyrazole, pyrazole, 1- (3-aminopropyl) imidazole, 2,2'-bis (4,5-dimethylimidazole), 4,5-dicyanoimidazole, 1,2-dimethylimidazole, 2 -ethylimidazole, histamine, ethyl ester of histidine, imidazole, N-methylthimidazole, 2-methylimidazole, 4-methylimidazole, benzothiazole, benzotriazole, isoquinoline, 1,10-phenanthroline, quinoline, 2-aminobenzimidazole, 2-aminoimidazole, aminopyrazine, 2-aminopyrimidine , 2-amino-4,6-dimethylpyrimidine, 2,6-diaminopyridine, L-histidine, 2-amino-1,3,4-triadiazole, 2,4,5-trimethyloxazole, trimethylpyrazine, benzimidazole, DL-histidine, pyrazine , Tetramethylpyrazine, trimethylpyrazine.
13. The method of claim 1, wherein the catalytic compound is selected from the group consisting of imidazole, N-methylimidazole, benzimidazole, pyridine, picoline (2-, 3- and 4-) lutidine (2,3-, 2,4-; 2,6-, 3,5-) collidine, quinoline, isoquinoline, thiophene, 3-thiophenecarboxylic acid, 3-thiopheneacetic acid, aminopyridine, 3-hydroxypyridine, dimethylaminopyridine, thiazole, methylthiazole, dimethyl thiazole, pyrazine, 3-amino-5, 6-dimethyl-1,2,4-triazine, pyrazole, 3,5-dimethylpyrazole, nicotinic acid, dicyanoimidazole, 2,4,5-trimethyloxazole, 2-amino-1,3,4-thiadiazole, L-histidine and 2 -aminopyrimidine.
14. The method of claim 2, wherein the pH of the system is about 9 or higher, the cyanide concentration is about 10 moles or lower and the catalyst compound is selected from the group consisting of imidazole, N-methylimidazole, 1,2-dimethylimidazole, pyridine, alpha-, beta -, gamma-picoline, 2-, 3-, 4-aminopyridine, 4-dimethylaminopyridine, thiophene, 3-thiophenecarboxylic acid, 3-thiopheneacetic acid, pyrazine, 2,4,6-trimethyltriazine, thiazole, L-histidine and 2- Aminopyrimidine.
15. The method of claim 3, wherein the pH of the system is about 8 to 9 and the catalyst compound is selected from the group consisting of pyridine, alpha, beta, gamma-picoline, di-2-pyridyl ketone, 2,2-dipyridyl, 3, 5-lutidine, 2,6-lutidine, 2,3-lutidine, N-methylimidazole, L-histidine and 2-aminopyrimidine.
16. The method of claim 4, wherein the pH of the system is about 9 or higher, and the catalyst compound is selected from the group consisting of pyridine, alpha, beta, gamma-picoline, di-2-pyridyl ketone, 2,2'-dipyridyl, 3 , 5-lutidine, 2,6-lutidine, 2,3-lutidine and N-methylimidazole.
17. The method of claim 6, wherein the pH of the system is about 2 to 3.
18. The process of claim 1, wherein the catalytic compound is 3-thiophenecarboxylic acid.
19. The process of claim 8, wherein the thiosulfate is present in the range of 0.1 to 0.25 mol, the copper (II) ion is present in the range of 0.01 to 0.15 mol, the ammonium ion (such as thiosulfate, hydroxide or sulfate) is present In the range of 0.5-4.8 mol and the catalytic compound is selected from the group consisting of pyridine, alpha, beta, gamma-picoline, di-2-pyridylketone, 2,2'-dipyridyl, 3,5-lutidine, 2 , 6-lutidine, 2,3-lutidine, imidazole, N-methylimidazole, thiophene, 3-thiophenecarboxylic acid, 3-thiopheneacetic acid, pyrazine-2,4,6-trimethyltriazine, thiazole, L-histidine and 2-aminopyrimidine.
20. The process of claim 9, wherein the pH of the system is about 10 to 12.
print version
Date of publication 14.03.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.