| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2241983
![]()
Method for the determination of rhenium and rhenium in the presence of molybdenum by the method of inversion voltammetry
The name of the inventor: Kolpakova NA (RU); Goltz LG (RU); Avgustinovich O.V.
The name of the patent holder: Tomsk Polytechnic University
Address for correspondence: 634034, Tomsk, Lenin Ave., 30, Tomsk Polytechnic University
Date of commencement of the patent: 2003.11.11
Use: in various branches of the national economy to determine the concentration of various metal ions in solutions. Essence: the method for the determination of metal ions involves the determination of rhenium and rhenium in the presence of molybdenum against a background of 1 M HNO 3 with the addition of 0.1 g of KNO 3 by inversion voltammetry (IVA), at which the electroconcentration of rhenium is carried out on a graphite electrode, followed by oxidation of the precipitate. The technical result of the invention is to increase the sensitivity by more than 2-3 orders of magnitude and reduce the analysis time.
DESCRIPTION OF THE INVENTION
The invention relates to analytical chemistry, namely to methods for determining metal ions, and can be used in hydrometallurgy, in various geological developments in prospecting and prospecting in the case of ore analysis, and in petrochemical industry to determine the concentrations of ions in solutions, ores and ore concentrates Rhenium by the method of inversion voltammetry (IVA).
The polarographic behavior of rhenium has been studied in acid solutions (HCl, HClO 4 , H 2 SO 4 , H 3 PO 4 , HNO 3 , acetic acid), in neutral solutions of potassium and sodium chlorides, and alkaline solutions [L.V. Borisova, A.N. Ermakov. Analytical chemistry of rhenium, M: Nauka Publishers, 1974, 315 pp.].
Rhenium behavior in hydrochloric and chloric acid solutions. The maximum height of the rhenium reduction wave is observed in 2-4.3 N HCl [Lingune JJJAm.Chem.Soc., 64, 1001, 2182 (1942); 65, 866 (1943)]. The potential of the half-wave E 1/2 for rhenium (VII) in 2 M HCl is -0.45 V, and in 4.2 M HCl -0.31 V. The disadvantage of this process is that the determination of rhenium is interfered by NO -3 ions, SO 2-4 , PO 3-4 , which distort the background line, and both molybdenum and tungsten, which are concomitant elements of rhenium in ores and ore concentrates.
Rhenium behavior in sulfuric and phosphoric acid solutions. Geyer showed [Geyer RZ anorg. Allgem. Chem., 263, 47 (1950)] that the number of reduction waves and their character varies with the concentration of H 2 SO 4 . The half-wave potential E 1/2 for rhenium (VII) in 3.5 M H 2 SO 4 is from 0.2 to -0.45 V. In this case, the anions Cl - , NO - , PO 3-4 , A and Mo, Fe, and Ti.
Rhenium behavior in neutral, alkaline and buffer solutions. The use of polarography in neutral and alkaline solutions is most suitable for the determination of small concentrations of rhenium [Rubinskaya T.Ya., Mayranovskii SG Electrochemistry, 7, 1403 (1971)]. Catalytic waves suitable for analytical purposes correspond to -1.2 to -1.6 V. In this process, the interfering effect is exerted by the ions Cl - , NO -3 , SO 24 , PO 3-4, and Mo, Cu, Zn , Cd, Pb, Bi, Se, Co and W.
Rhenium behavior in nitric acid solutions. The oscillographic method made it possible to observe the cathode wave of rhenium reduction. In 1M HNO 3, the half-wave potential for rhenium is -0.6 V. The detection limits were 10 -3 -10 -4 %. The interfering elements are Mo, Cu, Zn and W, and the anions of Cl- and others.
Polarographic methods for the determination of rhenium are known, which are divided into 2 groups:
A) Methods for the determination of large quantities (from 10 -3 % and higher), based on measurement of the waves of reduction of perrhenate;
B) methods for the determination of micro-quantities of rhenium in the range 10 -3 -10 -7 %, using catalytic currents and concentrating rhenium at the electrode in the form of a sparingly soluble oxide film.
The rhenium content in alloys from 2 × 10 -1 % and more is usually determined from the wave with E 1/2 = (-0.3) - (-0.4) B against a background of 2.5 M H 2 SO 4 . Smaller amounts of rhenium (up to 10 -3 %) in products of copper-molybdenum production are determined from the catalytic hydrogen wave with E 1/2 = -1.2 V against phosphate buffer solution (pH 7-8). The disadvantage of the method is that molybdenum is preliminarily separated in the form of sparingly soluble calcium molybdate by sintering the sample with CaO at 600-700 ° C for 2 hours. A detailed analysis of the analysis is given in [Kryukova TA, Sinyakova SI, Arefieva T. AT. Polarographic analysis. M., Goskhimizdat, 1959, p. 351].
Molybdenum concentrates are recommended to decompose and concentrate. HNO 3 followed by distillation of Re 2 O 7 from a sulfuric acid solution [Duca A., Stanescu D., Puscasu M. Studii si cercetari chim. Acad. RPRFil. Cluj, 6, 123 (1955); 13,197 (1962)]. Determination of rhenium is carried out against the background of a NaCl + Na 2 SO 3 solution (pH 11.3-11.5) at a molar ratio Mo: Re ![]() 200: 1 and E 1/2 = -0.45 V. The opening minimum is 1-2 μg Re / ml. The determination is also made after sample preparation, during which molybdenum, tungsten and other accompanying elements are separated from rhenium.
200: 1 and E 1/2 = -0.45 V. The opening minimum is 1-2 μg Re / ml. The determination is also made after sample preparation, during which molybdenum, tungsten and other accompanying elements are separated from rhenium.
A sensitive method is the inversion-voltammetric method for the determination of rhenium against a background of 4M H 3 PO 4 using an oscillograph polarograph and a mercury stationary microelectrode [Demkin AM, Sinyakova SI Sat. "Determination of trace impurities". Moscow, DNTP, 1968, p. 31] (prototype). Determination of rhenium is carried out according to the following procedure. An aliquot of 5-10 ml (4M H 3 PO 4 ) containing ~ 0.01-0.1 μg Re is placed in an electrolytic cell. Within 3 minutes, nitrogen is blown through the solution, the anodic polarization mode and the potential of a mercury microelectrode equal to 0.95 V are installed on an oscillograph polarograph. The wave is recorded at this potential. A necessary condition of the process is that rhenium in the samples is separated from molybdenum and tungsten. This condition is one of the drawbacks of this method.
The main task of the proposed solution is to determine rhenium and rhenium in the presence of molybdenum on an impregnated graphite electrode by the IVA method.
The objective is achieved by the fact that rhenium is electrochemically concentrated on various types of graphite electrodes (glassy carbon (SU), mercury-film (RP) or impregnated with graphite (IG)) followed by recording anode voltammograms. New in the method is that rhenium is accumulated in a stirred solution for 120-150 s at electrolysis potentials Ee = (-0.7) - (-0.0) V in the background: 1 M HNO 3 with the addition of 0, 1 g KNO 3 with the subsequent registration of anode peaks in the storage mode of shooting voltammograms at a sweep speed of 30-50 mV / s. The concentration of rhenium and rhenium in the presence of molybdenum is determined from the height of the anode peak in the range of potentials from 0.150 to 0.250 V with respect to the saturated silver chloride electrode (ADA).
In the prototype, the quantitative determination of rhenium is based on the reduction reaction and is possible only after its careful separation from molybdenum, which is often a concomitant element of rhenium in ores and ore concentrates.
In the proposed method, rhenium is oxidized for the first time on various types of graphite electrodes. IG, SU and RP were used as indicators (in the prototype, a mercury-drop electrode was used). The use of such electrodes is due to the high chemical and electrochemical resistance of graphite, a wide range of working potentials, and the ease of mechanical surface renewal and safety requirements. In the RP electrode, the consumption of mercury is significantly reduced, because Mercury is toxic.
The maximum value of the detected current is observed in the IG electrode. SU and RP electrodes and are suitable for use, however, because of the large residual current they were less convenient to operate than the IG electrode. The IG electrode was first used to determine rhenium and rhenium in the presence of molybdenum.
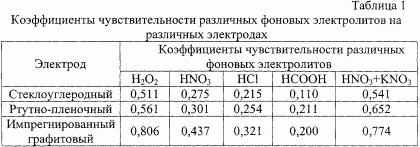
In the prototype, the use of a 4 M solution of phosphoric acid as a background is described. Under these conditions, only large amounts of rhenium can be determined (~ 10 -2 %). When determining the micro-quantities of rhenium under these conditions, it is necessary to isolate it from complex compositions, since most of the accompanying elements are interfering (for example, molybdenum, tungsten, and copper). The backgrounds of 1 M H 2 O 2 , or 0.5 M HNO 3 , or 1 M HCl, or 0.5 M HCOOH, or 1 M HNO 3 with the addition of 0.1 g KNO 3 offered in the claimed invention allow the determination of rhenium with a good Reproducibility. The use of backgrounds of 0.5 M HNO 3 and 1 M HCl is complicated by the fact that a rather large residual current was observed in the determination of rhenium, and when using backgrounds of 1 M H 2 O 2 and 0.5 M HCOOH, rhenium can not be determined in the presence of molybdenum, since On these backgrounds, the analytical signal of molybdenum muffles the analytical rhenium signal. The highest sensitivity coefficient was observed against a background of 1 M HNO 3 with the addition of 0.1 g of KNO 3 . The use of this background and solves the problem of the joint determination of rhenium and molybdenum. The detection limit is 10 -6 -10 -2 % (in the prototype 10 -3 -10 -2 %).
The results of the use of various electrodes on different backgrounds are given in Table. 1. From Table 1 it is seen that the IG electrode has the highest sensitivity coefficients. The same picture is observed if the determination is made against the background of 1 M HNO 3 with addition of 0.1 g of KNO 3 , in addition, rhenium in the presence of molybdenum can be quantitatively determined against this background. Thus, we proposed the determination of rhenium and rhenium in the presence of molybdenum on an IG electrode and against a background of 1 M HNO 3 with the addition of 0.1 g of KNO 3 .
Another characteristic feature is the established conditions for electrochemical accumulation: the electrolysis potential is E = (-0.7) - (-0.0) V. Experimental data have shown the dependence of the rhenium oxidation current on E e (Table 2). The value of the anode current increased and reached a maximum value in the potential range (-0.7) - (-0.0) V. The use of pre-electrolysis at potential values of -0.8 ± 0.1 V allows one to register voltammograms with a clearly expressed maximum. This makes it possible to increase the accuracy and resolution of the method.
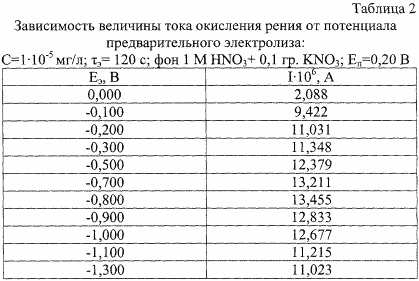
Pre-electrolysis time ( ![]() E ) is selected depending on the concentration of the substance to be determined. The maximum value of the oxidation current is reached when
E ) is selected depending on the concentration of the substance to be determined. The maximum value of the oxidation current is reached when ![]() E , equal to 120-150 s. When
E , equal to 120-150 s. When ![]() E <120, the sensitivity of the determination decreases and the determination error increases, and when
E <120, the sensitivity of the determination decreases and the determination error increases, and when ![]() E > 150 the speed decreases.
E > 150 the speed decreases.
Important for the determination of rhenium is the choice of the sweep rate of the potential. The optimum speed is 30-50 mV / s. Increasing the speed of more than 50 mV / s increases the sensitivity, but the residual current increases and the resolving power of the method decreases. Using a velocity of 30 mV / s significantly reduces the value of the anode current and decreases the sensitivity of the determination of rhenium.
Thus, the established conditions for the first time allowed the quantitative determination of rhenium and rhenium in the presence of molybdenum on the basis of the electrooxidation reaction. To increase the sensitivity of the determination, preliminary concentration of rhenium on the surface of graphite electrodes was used. The proposed voltammetric method significantly improved the metrological characteristics of the rhenium analysis, increased the sensitivity of the determination (1 · 10 -6 mg / kg), which is 3-4 orders of magnitude lower compared to the prototype, the analysis time does not exceed 120-150 s versus 30 minutes. Definition does not interfere with molybdenum, tungsten and copper, which are accompanying elements.
As a prototype, a method for the determination of rhenium by the current-voltage curves of the electroreduction Re (7 +) -> Re (4+) was chosen. We have proposed the determination of rhenium by the IVA method, in which the electroconcentration of rhenium on a graphite electrode is carried out, followed by oxidation of the precipitate. The analysis was performed on a STA-1 instrument (Tomsk, Russia). The method chosen by us makes it possible to significantly expand the range of detectable concentrations from 10 -3 -10 -2 to 10 -6 -10 -2 %), but also allows to simplify the hardware design of the process. In addition, this method makes it possible to determine rhenium in the presence of molybdenum, which solves the questions of sample preparation for analysis.
Examples of specific implementation
Example number 1 . The measurements were carried out on artificial mixtures. 10 ml of the background electrolyte is placed in a quartz cup. Without interrupting the mixing, electrolysis of the solution is carried out under the condition: Ee = -0.800V, ![]() E = 120 s. The volt-ampere curve of the electro-oxidation is removed at a scanning speed of 40 mV / s, starting from the potential E init = 0.000 V. Absence of peaks indicates a purity of the background. Then 0.05 ml of a standard sample (CO) of rhenium is added and the electrochemical concentration of the precipitate is carried out under similar conditions. The peak for the indicated concentration of the substance is recorded in the potential range from 0.150 to 0.250 V (relative to the pH). A 0.05 ml rhenium reduction additive was made and an analytical signal was recorded again. By the difference in the currents of the rhenium peaks, the concentration of rhenium in the solution was calculated. Determination of rhenium in the presence of molybdenum is carried out analogously, only molybdenum is added to the background electrolyte (from 0.05 to 5 ml of molybdenum CO). It has been established that rhenium can be quantified at a 100-fold excess of molybdenum (compared to rhenium). Rhenium was prepared by diluting 0.05 g of pure rhenium powder in 50 ml of a solution containing 25 ml of concentrated HNO 3 and tri-distilled water. CO molybdenum was obtained in the same way. A background electrolyte was prepared by diluting concentrated nitric acid (10 ml of conc. HNO 3 in a 100 ml volumetric flask) and adding 0.1 g of KNO 3 . The measurement error is on the order of 1-5%.
E = 120 s. The volt-ampere curve of the electro-oxidation is removed at a scanning speed of 40 mV / s, starting from the potential E init = 0.000 V. Absence of peaks indicates a purity of the background. Then 0.05 ml of a standard sample (CO) of rhenium is added and the electrochemical concentration of the precipitate is carried out under similar conditions. The peak for the indicated concentration of the substance is recorded in the potential range from 0.150 to 0.250 V (relative to the pH). A 0.05 ml rhenium reduction additive was made and an analytical signal was recorded again. By the difference in the currents of the rhenium peaks, the concentration of rhenium in the solution was calculated. Determination of rhenium in the presence of molybdenum is carried out analogously, only molybdenum is added to the background electrolyte (from 0.05 to 5 ml of molybdenum CO). It has been established that rhenium can be quantified at a 100-fold excess of molybdenum (compared to rhenium). Rhenium was prepared by diluting 0.05 g of pure rhenium powder in 50 ml of a solution containing 25 ml of concentrated HNO 3 and tri-distilled water. CO molybdenum was obtained in the same way. A background electrolyte was prepared by diluting concentrated nitric acid (10 ml of conc. HNO 3 in a 100 ml volumetric flask) and adding 0.1 g of KNO 3 . The measurement error is on the order of 1-5%.
Example number 2 . 0.1 g of catalyst powder containing rhenium was dissolved in 15% HNO 3 in a 100 ml flask for 15-20 minutes with stirring. 10 ml of the background electrolyte was placed in a quartz cup, an aliquot of 1-1.5 ml of the resulting catalyst solution was added. The volt-ampere curve of the electro-oxidation of rhenium was taken off. A rhenium reduction additive of 1 ml was made and an analytical signal was recorded again. By the difference in the currents of the rhenium peaks, the concentration of rhenium in the solution was calculated. The peak of the rhenium current was recorded at a potential E = 0.200 V. The concentration of rhenium was ~ 65%. The rhenium and the background electrolyte are prepared in the same way as in Example No. 1.
Thus, the ability of a quantitative analysis of rhenium for the peaks of its oxidation on an IG electrode was established for the first time (in a prototype, the quantitative determination of rhenium is carried out by recovery waves on a mercury dropping electrode). Significantly increased the sensitivity of the definition (more than 2-3 orders of magnitude). The conditions used in the prototype do not allow the analysis of rhenium in the presence of molybdenum.
The proposed method is simple, eliminates the use of toxic mercury, does not require much labor, a large number of reagents and can be used in any chemical laboratory with computerized analyzers such as CTA, TA or polarograph. The proposed method can be used to determine rhenium and rhenium in the presence of molybdenum in ores and ore concentrates.
Method for the determination of rhenium and rhenium in the presence of molybdenum by the method of inversion voltammetry.
CLAIM
A method for the determination of rhenium and rhenium in the presence of molybdenum by the method of inversion voltammetry, consisting in the fact that rhenium is transferred from a sample to a solution and a voltammetric determination is carried out, characterized by the accumulation of rhenium on an impregnated graphite electrode in a stirred solution for 120-150 s at potentials Electrolysis (-0.7 ÷ -1.0) in a relatively saturated silver chloride electrode against a background of 1 M HNO 3 with the addition of 0.1 g of KNO 3 with subsequent registration of anode peaks in a storage mode of voltammograms at a potential sweep rate of 30-50 mV / S and the concentration is determined from the height of the peak in the range of potentials of 0.150 - 0.250 V by the method of additives of certified mixtures.
print version
Date of publication 14.03.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.