| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2165448
![]()
DETERGENT AND ANTI-CORROSION DEPOSIT FOR FUEL AND FUEL ON ITS BASIS
The name of the inventor: ZHERMANO Laurent (FR); RAUL GI (FR); Eber Daniel (FR)
The name of the patentee: ELF ANTAR FRANCE (FR)
Patent Attorney: Egorova Galina Borisovna
Address for correspondence: 129010, Moscow, ul. Bolshaya Spasskaya 25, page 3, LLC "Gorodissky and Partners", Egorova G.B.
Date of commencement of the patent: 1997.09.17
The invention relates to a bifunctional additive with detergent and anticorrosion functions, which when added to automotive fuels significantly reduces the problems associated with corrosion of certain engine parts and the formation of deposits. The detergent and anticorrosive additive for automotive fuels, in particular for gas oil type fuels, includes amide or imide groups. It is prepared by mixing 60-90% by weight of compound A, which is at least one carboxylic polyalkylene compound, a diene or anhydride having an average molecular weight of from 200 to 3000, 0.1 to 10% by weight of compound B, which is at least carboxyl A monobasic or anhydride containing 1-6 carbon atoms per chain and 10-30% of compound C being at least one primary polyamine of the general formula H 2 N - [- (CHR 1 - (CH 2 ) p - CHR 2 ) N - NH] m - H, with the mass ratios A / B / C corresponding to 1 / (0,1-1) / (1-3), A / B / C can never be 1/1/1. The additive significantly increases the detergent and anti-corrosion properties of fuels, reduces the emission of contaminants and smoke.
DESCRIPTION OF THE INVENTION
The present invention relates to a bifunctional additive with detergent and anticorrosive functions that when added to automotive fuels significantly reduces the problems associated with corrosion of certain engine parts and the formation of deposits.
In essence, the use of traditional fuels without detergent and anti-corrosive additives promotes the accumulation of deposits in the fuel supply system, in particular at the level of the injectors, which are being smeared, even in the combustion chamber, due to the presence of polar aromatic compounds and traces of lubrication.
Accumulation of deposits worsens the volatility of the fuel, which causes an increase in consumption, an increase in emissions of contaminants and smoke, in particular, significantly higher during acceleration, and finally, noise amplification that can not be neglected.
In order to solve the problem of engine fatigue, it is possible to clean the jammed components periodically, in particular nozzles, but this process becomes very expensive with time.
Another way to reduce resinous deposits in engines and in particular nozzles is to add detergent-type additives to the fuel, the function of which is to be absorbed on metal surfaces to prevent the formation of deposits (preventive effect) and / or to remove deposits that have already formed In addition, among the additives used in fuels as well as in lubricants, in particular, the condensation products of succinic acid polyalkynylanhydrides and polyamides such as tetraethylenepentamine described in US Pat. No. 3,272,892 are known. If these additives give good results The results in terms of limiting the formation of deposits on new injectors, they continue to be ineffective for cleaning already molded injectors.
A detergent-dispersant additive for automotive fuels is known, obtained by the reaction of polyamidoamines with polyalkylenesuccinic acid anhydrides. In other words, the reaction of A with molecules of amidoamines CBC is carried out. A contains 2-10 carbon atoms per linear or branched alkylene group with an average molecular weight of 300-10000; Compound B is a mono- or dicarboxylic acid or an anhydride thereof, for example methacrylic acid, acrylic acid, maleic or succinic anhydride; Compound C is, for example, a primary amine, including a polyamine selected from the group: polyethyleneamine, diethylene triamine, triethylenetetramine, tetraethylenepentamine, etc. Compound A is located mainly on the free NH 2 groups of two C aminoamine CBC (US Pat. No. 5,034,018).
The task of this additive is only the restriction of contamination at the level of the injectors.
It is an object of the present invention to provide a bifunctional additive with detergent and anticorrosive properties that is compatible with other commonly used additives, in particular gas oil, which allows to reduce and even inhibit the formation of deposits at the level of the nozzles while limiting corrosion phenomena and maintaining a high dispersion.
The subject of the present invention is thus a bifunctional additive for automotive fuel, in particular a gas oil type with detergent and dispersion properties, comprising amide or imide groups obtained by condensing the compound C formed by the primary polyamine with compound A formed by at least one A diacid or an anhydride, and a compound B which is at least one carboxyl compound, monobasic or anhydride linear or branched, said additive being characterized in that it is prepared by mixing 60-90% by weight of compound A containing 2-20 Carbon atoms per linear or branched alkylene group having an average molecular weight of from 200 to 3,000, 0.1 to 10% by weight of compound B having 1 to 6 carbon atoms per chain and 10 to 30% of compound C of general formula (I)
H 2 N - [- (CHR 1 - (CH 2 ) p -CHR 2 ) n -NH] m -H, (1)
In which R 1 and R 2 , identical or different, are hydrogen or a hydrocarbon group containing 1 to 4 carbon atoms, n is an integer from 1 to 3, m is an integer from 1 to 10, and p is an integer, Equal to 0 or 1.
According to the invention, compounds A, B and C are used in molar ratios A / B / C, preferably 1 / (0.1-1) / (1-3) and necessarily differing from 1/1/1. In fact, there is always an excess of polyamine in the selected composition, which leads to the fact that some number of terminal NH 2 groups of the polyamine C remains free. Preferably, the C / A molar ratio varies from 1.3 to 2.0, and the molar ratio B / A varies from 0.1 to 0.8.
In comparison with the known additives, the combination of mono- and dicarboxylic compounds in addition to the polyamine contributes to the detergency and anticorrosive effect of the additives of the invention. It corresponds to the synergistic effect of these three compounds.
The average molecular weight of the carboxyl polyalkylene compounds of the present invention varies preferably from 200 to 2000, and most often from 200 to 1500. These compounds are well known in the art; In particular, they are obtained by reacting at least one α -olefin or at least one chlorinated hydrocarbon, both linear or branched, with maleic acid or anhydride. This olefin or this chlorinated hydrocarbon typically contains from 10 to 150 carbon atoms, and preferably from 15 to 80 carbon atoms and most often from 20 to 75 carbon atoms in their molecule. The olefin may also be an oligomer, such as a dimer, trimer or tetramer, or a lower olefin polymer containing 2-10 carbon atoms, such as ethylene, propylene, n-butene, isobutene, n-hexene, n-octene-1, methyl -2-heptene-1 and propyl-2-propyl-5-hexene-1. Without departing from the scope of the present invention, mixtures of several olefins or several chlorinated hydrocarbons could be used.
In a preferred embodiment of the invention, the polyalkylene carboxyl compounds are selected from polyalkylene derivatives of succinic acids and anhydrides, wherein the anhydride number varies from 0.5 to 1.2 milliequivalents of KOH per gram of product.
Among succinic anhydrides, the preferred anhydrides are succinic n-octadecenyl anhydride, succinic anhydride succinic anhydride and succinic polyisobutenyl anhydrides and all succinic anhydrides with a weight average molecular weight ranging from 200 to 1500.
In a preferred embodiment of the invention, compound B is preferably selected from the group consisting of methacrylic acid, acrylic acid, maleic anhydride, succinic anhydride, malonic acid, fumaric acid and adipic acid.
Among the primary polyamines of formula (I), polyamines selected from the group: diethylene triamine, dipropylene triamine, triethylenetetramine, tetraethylenepentamine and substituted derivatives thereof.
Mixing of compounds A, B and C can be carried out in any order. However, in the preferred embodiment, the substance C is added, i.e. A primary polyamine of formula (I) to a mixture of compounds A and B, i.e. To a mixture of carboxylic hydrocarbons. The process is usually carried out by gradually adding polyamine C to a solution in an organic solvent of this mixture of carboxylic hydrocarbons at ordinary temperature, then the temperature is usually adjusted to 65-250 ° C. and preferably to 80-200 ° C. The organic solvent required for dissolution is selected by the boiling point component 65-250 ° C and the ability to remove the water formed during the condensation of the polyamine and the A + B mixture by azeotropic distillation of the water / solvent mixture. The solvent is preferably selected from the group consisting of benzene, toluene, xylenes, ethylbenzene and technical fractions of hydrocarbon distillation, for example hydrocarbons distilled at a temperature of 190-209 ° C. and containing 99 wt. % Of aromatic compounds. Naturally, without departing from the scope of the present invention, it is possible to use a mixture of solvents, in particular a mixture of xylenes or a mixture of xylene / alcohol, in particular ethyl-2-hexanol, on the one hand, to facilitate uniformity of the medium, and, on the other hand, to improve Reaction kinetics. After completion of the addition of the primary polyamine C, the heating is maintained at reflux until the water is completely removed, usually for 0.5 to 7 hours, preferably 1 to 5 hours.
The second subject of the invention is a fuel consisting mainly of a medium run from a crude oil straight-run fraction at 150-400 ° C, or any other fuel with a cetane number greater than or equal to 30, and a smaller portion of the detergent (s) and the anticorrosive (s) Bifunctional additive (s) according to the first subject of the invention.
In a preferred method of such a fuel, the content of the detergent and anticorrosion additive (s) is greater than 50 ppm, preferably 60 to 600 ppm.
According to the present invention, at least one additive of a group of oily additives, cetane number improvers, desemulsifying additives and odor-modifying additives can be added to said fuel.
The following examples are intended to illustrate the invention without limiting its scope.
Example I
In the present example, the preparation of several samples of detergent and anti-corrosion bifunctional additives according to the invention is described.
These samples of the invention are designated X i , and the comparative examples C i , where i corresponds to a numbering that allows them to be distinguished.
The composition of these samples is given in Table. 1.
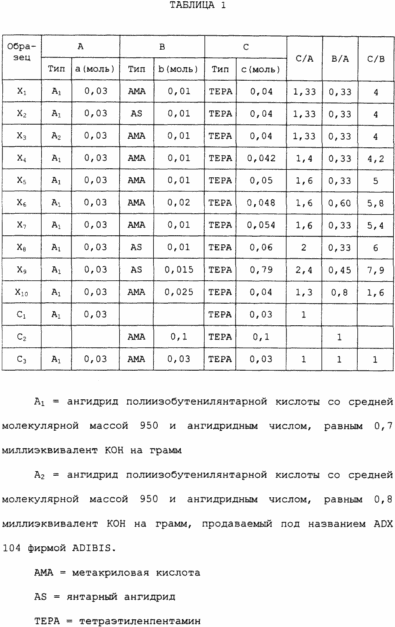
Samples X i , given in Table. 1 was prepared according to the following procedure.
Enter alternately into a four-necked flask of 250 ml of moles of polyisobutenylsuccinic anhydride A, L of Compound B, 25 ml of ethyl 2-hexanol and 25 ml of xylene. The mixture is stirred and heated to 100 ° C. until a homogeneous medium is obtained, after which it is added over about 5 minutes with moles of tetraethylene pentamine or TEPA, C. All are held together at the same temperature under reflux for three to four hours until a constant volume of recoverable Water (1.05 ml). The compounds obtained have two characteristic absorption bands of the IR spectrum of imide groups at 1700 cm -1 and amide groups at 1670 cm -1 .
For comparative examples, C 1 , C 2 and C 3 act as in the previous case for samples X i , but the ratio of components A, B and C is changed. Infrared spectroscopy shows characteristic absorption bands of imides at 1700 cm -1 (intensive) and Amides at 1670 cm -1 (weak).
Example II
This example demonstrates the increased detergency of the samples of the invention, depending on the relative contents of A, B and C after addition to diesel fuel. This example has the aim of emphasizing the synergistic effect obtained with the combination according to the invention.
The gas oil used is a diesel fuel with the following main characteristics:
- density at 15 o C 0.836 kg / l
- initial distillation temperature 174 ° C
- Final distillation temperature 366 ° C
Cetane number 53
- sulfur content of 0.24% by weight
The tests were carried out only on diesel fuel or with one of the additives Xi of the invention or comparative detergents C i at a mass active matter content of 175 parts per million.
These tests consist in acting according to the order of the motor test, such as described in the literature, published by SAE (Society for Automotive Engineers) in SAE # 922184, 1992. They are conducted on two Kubota Z 600-B generator sets with a drive From four-stroke two-cylinder diesel engines with indirect injection of 570 cm 3 .
Each test is carried out for 6 hours under the following conditions:
- engine mode; 3000 rpm;
Load: 2/3 of the maximum load.
At the beginning of each test, the engines are equipped with new injectors, the flow rate of which was preliminarily measured when they were installed at different nozzle lift heights. At the end of each test, the nozzles are removed and their costs measured at the same needle lift heights. The efficiency of the detergent additives studied is compared based on the percentage of their residual flow (% dr), calculated by the following formula.

In Table. II shows the results obtained.
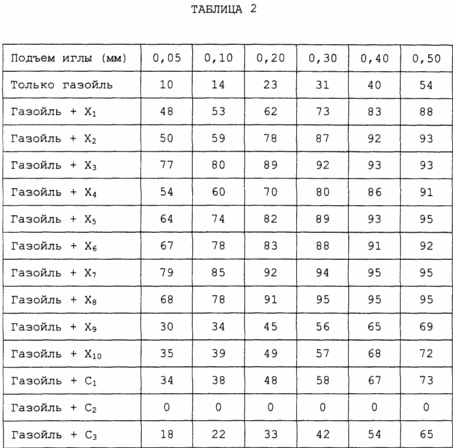
As can be seen from Table I, the additives of the invention give higher residual costs than those obtained using only gas oil and gas oil with comparable detergent additives.
Example III
The purpose of this example is to demonstrate the effectiveness of the additives of the invention for cleaning already molded injectors (eliminating the effect) in comparison with the additives C in accordance with the procedure described in Example II. Prior to each test, the nozzles were preliminarily coated with gas oil without additive for 6 hours in accordance with the procedure described in Example II.
Residual costs after the phase of scouring only gas oil are given in line 1 of Table. II.
The effectiveness of the additives for cleaning already molded nozzles is calculated using the following formula: 
Data on the effectiveness of additives relative to the purification of polluted nozzles, given in Table. III, are given for each lifting of the needle; They show the superiority of the additives of the invention.
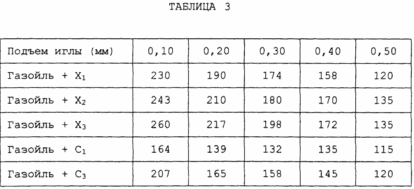
Example IV
The purpose of this example is to show the superiority of the additives of the present invention with respect to the comparative additives C.
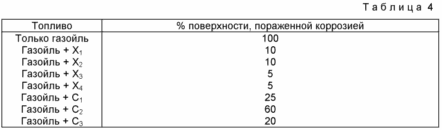
Corrosion tests consist of determining the anticorrosive effect of additives in gas oil on samples of ordinary polished steel in the presence of artificial seawater in accordance with ASTM D665, for 24 hours at 60 ° C. They are expressed in% of the surface affected by corrosion.
As the results of Table. IV, the additives of the invention have high anticorrosion properties superior to the anticorrosive properties of the known materials.
Comparative tests
Two additives were made under the conditions described in the examples of patent US 5.034.018 on page 22, starting with line 38, namely:
CBC 1 = Amidoamine 1 = 2 equivalents of TEPA (tetraethylenepentamine in reaction with 1 equivalent of methyl acrylate).
CBC 2 = Amidoamine 2 = 1.3 equivalents of TEPA in reaction with 0.8 equivalents of methyl acrylate.
Each CMS amidoamine was reacted with polyisobutenylsuccinic anhydride or PiBSA 1 according to the present application at a molar ratio of 1/1. The two products respectively designated X and Y.
The technique used
CBC 1 = Amidoamine 1 = Methyl acrylate + TEPA (1/2)
A 8.6 g (1.1 mole) methyl acrylate and 37.8 g (0.2 mole) of tetraethylenepentamine are alternately added to a four-neck four-necked flask (equipped with a thermometer, a mixer, a filling funnel and a nitrogen accelerator at ambient temperature.) The temperature is raised to 52 ° C, the medium is colorless , Clear and uniform.The mixture was heated to 140 ° C. for 3 h 30 min and methanol, characteristic of the amidation reaction, was recovered.
Amidoamine 1 is obtained as a viscous transparent orange liquid, homogeneous both in hot and cold state.
CBC 2 = Amidoamine 2 = Methyl acrylate + TEPA (0.8 / 1.3)
A 10.32 g (0.22 mole) methyl acrylate and 36.29 g (0.192 mole) of tetraethylenepentamine are alternately added to a four-necked four-necked flask (equipped with a thermometer, a mixer, a filler and a nitrogen blower) at ambient temperature. The temperature is raised to 55 ° C., while the medium is colorless, transparent and homogeneous. The mixture is heated to a temperature of 140 ° C. for 3 hours 30 minutes and methanol is obtained, characteristic of the amidation reaction.
Amidoamine 2 is obtained in the form of a viscous transparent pale yellow liquid, homogeneous both in hot and cold state.
X = PiBSA + amidoamine 1
In a 500 ml four-necked flask (equipped with a thermometer, a mixer, a filler and a nitrogen blower), 80 g of polyisobutenylsuccinic anhydride (with an anhydride number of 0.66 milliequivalents per gram) are added at ambient temperature. The medium is brought to 120 ° C. and 22.8 g (1 equivalent) of amidoamine 1 and 61.7 g of xylene (solvent) are alternately added to obtain the final product with 50% of the active substance. Medium brown-orange turbidity color is kept for two hours for the outflow of xylene (until a theoretical amount of water). The reaction product DE 1836 is obtained as a 50% solution in xylene.
Y = PiBSA + amidoamine 2
The process is carried out as described above, but using 18.8 g of amidoamine 2 and 57.7 g of xylene, all other conditions are maintained identical.
The products were tested in accordance with reference D at a dose of 170 ppm of active substance in gas oil meeting the European standard EN 590. Products were tested immediately after manufacture and after 1 month of storage at ambient temperature. The test conditions corresponded to those described in the application, with the exception of the KUBOTA engine, which was replaced by a 4-cylinder LOMBARDINI LDW 2004 indirect injection engine with a volume of 2068 cm 3 .
Both products were tested for their infrared and detergent properties relative to the product described in Example 1 of this application and labeled D. Products were tested immediately after their manufacture and after 1 month of storage at ambient temperature.
These results show that the products described in US Pat. No. 5,010,018 are unstable and vary with time both in appearance and in efficiency, the claimed products according to the invention are more effective.
CLAIM
1. A detergent and anticorrosive additive for automotive fuels, in particular gas oil type fuels, containing amide or imide groups obtained by condensing compound C, which is a primary polyamine, with compound A being at least one polyalkylene carboxylic compound, diacid or anhydride, and A compound B which is at least one linear or branched carboxyl compound, a monoacid or an anhydride, characterized in that it is prepared by reacting a compound C of formula I
H 2 N - [- (CHR 1 - (CH 2 ) p -CHR 2 ) n -NH] m -H,
In which R 1 and R 2 , identical or different, are hydrogen or a hydrocarbon group containing 1 to 4 carbon atoms;
N is an integer from 1 to 3;
M is an integer from 1 to 10;
P is an integer equal to 0 or 1,
With a mixture of two compounds A and B contained in an organic solvent having a boiling point of 65 to 250 ° C, compound A is a polyalkylene carboxylic compound having from 2 to 20 carbon atoms per linear or branched alkenyl group and having an average molecular weight of from 200 to 3000 , And compound B is selected from the group consisting of methacrylic acid, acrylic acid, maleic anhydride and succinic anhydride, the molar ratios A / B / C being 1, (0.1-1) / (1-3), wherein A / B / C can never be 1/1/1, the C / A molar ratio varies from 1.3 to 2 and the B / A molar ratio varies from 0.1 to 0.8.
2. An additive according to claim 1, characterized in that the average molecular weight of the polyalkylene carboxylic compounds A varies from 200 to 2000 and preferably from 200 to 1500.
3. An additive according to claim 1 or 2, characterized in that the polyalkylene carboxylic compounds are selected from succinic acids and anhydride derivatives of a polyalkylene, wherein the anhydride number is 0.5 to 1.2 milliequivalents of KOH per 1 g of compound.
4. Additive according to any one of claims 1 to 3, characterized in that the succinic anhydrides are selected from the group formed by succinic n-octadecenyl anhydride, succinic anhydride and succinic polyisobutenyl anhydrides, the weight-average molecular weight of all succinic anhydrides ranging from 200 to 1500.
5. An additive according to any one of claims 1 to 4, characterized in that the primary polyamines are polyamines from the group formed by diethylenetriamine, dipropylene triamine, triethylenetetramine, tetraethylenepentamine and their substituted derivatives.
6. Fuel consisting, for the most part, of at least an average run from the crude oil direct distillation fraction at 150-400 ° C, or any other fuel with a cetane number greater than or equal to 30, and a smaller portion of the additive according to any one of .1-5.
7. A fuel according to claim 6, characterized in that it contains at least 50 ppm , preferably 60-600 ppm, of the detergent (s) and anticorrosive additive (s).
print version
Date of publication 07.04.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.