| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2160160
![]()
CATALYST AND METHOD FOR PRODUCING LIQUID HYDROCARBONS FROM DIMETHYL ETHER
Name of the inventor: Baybursky VL .; Winz VV .; Genkin VN .; Genkin MV .; Lischiner I.I .; Malov OV .; Mortikov ES .; Dolinsky SE
The name of the patentee: Baybursky Vladimir Leonovich; Winz Viktor; Genkin Vladimir N.; Genkin Mikhail; Lischiner Joseph Izrailevich; Malova Olga; Mortikov Eugene S.; Sergei Dolinsky Erikovich
Address for correspondence: 121609, Moscow, Autumn Blvd., 11, company "VIS"
Starting date of the patent: 1999.10.22
The invention relates to a process for producing liquid hydrocarbon enriched iso- and cycloparaffins, which may be used as an additive in the production of high-octane gasoline containing aromatic hydrocarbons of not more than 30 wt.%. Liquid hydrocarbons from dimethyl ether is prepared using a catalyst based on a crystalline pentasil-type aluminosilicate with an SiO 2 / Al 2 O 3 = 25-100, containing 0.05-0.1 wt.% Sodium oxide, and a binder component, which further comprises an oxide zinc and rare earth oxides in the following ratio, wt%:. Zn0 0,5-3,0, REE oxides 0.1-5.0, 65-70 crystalline aluminosilicate, a binder else. The catalyst was activated in air at 540-560 ° C. The process is carried out at a pressure of 0.1-10 MPa, a temperature of 250-400 ° C, the volumetric hourly space velocity of 250-1100 h -1.
DESCRIPTION OF THE INVENTION
The invention relates to methods for the preparation of hydrocarbons from dimethyl ether, in particular a hydrocarbon mixture rich iso- and cycloparaffins. Such liquid hydrocarbons can be used as an additive to produce high-octane gasoline containing aromatic hydrocarbons of not more than 30 wt.%.
The selectivity of the conversion of dimethyl ether into valuable hydrocarbons (LPG, motor gasoline, aromatic hydrocarbons and others.) Is determined by the properties of the catalyst and process conditions. It is known that effective catalysts for the conversion of dimethyl ether into a mixture of hydrocarbons compositions are metal components and inorganic zeolites, in particular decationized pentasil H-ZSM-5, H-ZSM-11. [Proc. Int. Zeolite Conf. , 6th. Meeting Date 1983, 316-324, 489-96: Guildford, UK. (English) in 1984; S.African ZA 8401683 A 19841128].
A method of producing liquid hydrocarbons or a mixture of C 2+ olefins from dimethyl ether to the modified zeolite catalyst or a metallosilicate at 300-700 o C, a pressure of 0.1 MPa, and a mass hourly space velocity of 2.3 h -1 [S.African ZA 9004752 A ; 19920226]. With an increase in temperature from 300 to 600 o C increases the selectivity of C 2 -C 4 olefins. The total yield of olefins reaches 74-75%, the rest - liquid hydrocarbons.
A method of producing isoparaffinic hydrocarbons from dimethyl ether described in [Pat. US 4579999, 1986].
According to said patent dimethylether vysokokremnezemnyh on ZSM-5 catalyst in the first stage is converted into a mixture of C 2 olefins and C 4 -C 5+ hydrocarbons. The resulting mixture is directed to oligomerization of olefins using acidic zeolite catalyst sredneporistogo. The second process step is carried out at elevated pressure and moderate temperatures. Is provided and light hydrocarbons is recycled to the first stage of the process. The disadvantage of this method is its multistage.
The closest to the claimed method is a process for producing olefins C 2 -C 5 hydrocarbon liquid and at a pressure of from 0.1 to 5.0 MPa, a temperature of 360 o C and a mass hourly space velocity of 1.65 h -1 [Pat.SShA 3,894,106, 1975]. The catalyst provides a catalyst system comprising 65% HZSM and 35% U Al 2 O 3. The disadvantages of this method are the formation of large amounts of gaseous hydrocarbons (25-40 wt.%) And a high yield of aromatic hydrocarbons (45 wt.%).
The technical result of the proposed invention is to increase the yield of high-octane component of motor fuels with a low content of aromatic hydrocarbons from dimethyl ether, or gases containing dimethyl ether.
Said technical result is achieved in that in the preparation of liquid hydrocarbons from dimethyl ether using a catalyst based on a crystalline pentasil-type aluminosilicate with an SiO 2 / Al 2 O 3 = 25-100, containing 0.05-0.1 wt.% Sodium oxide, and a binder, which further comprises zinc oxide and oxides of rare earth elements in the following ratio, wt.%:
- ZnO - 0,5 - 3,0
- oxides of rare earth elements - 0.1-5.0
- crystalline aluminosilicate - 65 - 70
- binder - the rest
The catalyst was activated in air at a temperature of 540 - 560 o C.
The process is carried out at a pressure of 0.1-10 MPa, a temperature of 250-400 o C, the volume of raw material feed rate of 250-1100 h -1.
The observed technological effects increase the yield of liquid hydrocarbons due, apparently acid modifying properties and catalytic activity of the zeolite component and possibly a promoting effect lanthanide oxides on the surface of the zeolite crystallites.
Zeolites used in the composition of the proposed catalyst are the domestic counterparts pentasil CVM, TSVMSH (both TU 38.401528-85), WHC and PPM (TU 38.102168-85) containing 0.2-0.5 mol.% Na 2 O and obtained by direct synthesis (WHC) and the exchange of the original Na-form of the zeolite in the H + - or NH 4+ form.
The synthetic aluminosilicates, alumina can be used as a binder.
REE oxides formed on the surface of the catalyst with calcination at 550 o C zeolite impregnated industrial concentrate REE nitrates. For the modification of zeolites as a source of REE REE concentrate used industrial nitrate, containing in 1 liter of 200 g of the following composition REE oxides, they say. %: CeO 2 - 48; the sum of La 2 O 3, PrO 3 and Nd 2 O 3 - 52 or an aqueous solution of neodymium nitrate.
The following examples confirm the effectiveness of the proposed method for producing desired products.
Example 1. WHC zeolite synthesized with SiO 2 / Al 2 O 3 equal to 42, using monoethanolamine. The hydrogen form of the zeolite with a predetermined residual content of Na 2 O (0,05-0,1 wt.%) Obtained by double exchange of Na in 30% solution of ammonium nitrate, followed by drying and calcining for 3 hours at 500-550 o C. Zn oxide is introduced into the zeolite in the ammonium form of sharing it with an aqueous solution of zinc nitrate. Calculated amount of Nd 2 O 3 deposited on the zeolite by impregnation Residue from an aqueous solution of neodymium nitrate. Zeolite with a given content of oxides obtained by calcination of the impregnated sample at 500-550 o C. The calculated amount of calcined zeolite is mixed with a binder - aluminum hydroxide (p.m.p.p. - 70%), formed by extrusion. Catalyst granules (2x2 mm) were dried at 100 o C for 2 hours, and then activated by calcination in air at 550 o C for 3 hours. The catalyst given below, wt.%:
- ZnO - 2,0
- Nd 2 O 3 - 1.0
- Zeolite WHC - 67.0
- Al 2 O 2 - 30
Example 2. The catalyst was prepared as in Example 1 with the difference that in step the ammonium form zeolite impregnation instead of the neodymium nitrate aqueous solution, an aqueous solution concentrate composition of lanthanide (calculated as oxides obtained) GeO 2 - 48; the amount of La 2 O 3, PrO 3 and Nd 2 O 3 - 52% mole. The catalyst given below, wt.%:
- ZnO - 2,0
- REE oxides - 1.0
- Zeolite WHC - 67.0
- Al 2 O 3 - 30
Examples 3-18. The catalysts prepared in Examples 1, 2, is used to produce hydrocarbons from dimethyl ether in an isothermal flow reactor with a catalyst loading of 10 g at a reactor temperature of 250-400 o C, a pressure of 0.1-10.0 MPa, volumetric feed rate of the raw material gas 250 -1100 h -1. Liquid and gaseous reaction products are analyzed by chromatographic methods. The raw materials used pure dimethyl ether (raw material 1) or a gas mixture of the following composition containing dimethyl ether (raw material 2), see. Table. 1.
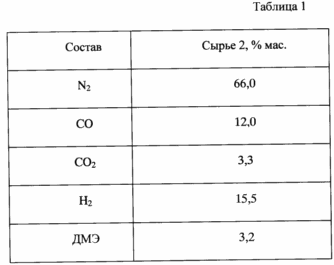
The results are shown in Table. 2.
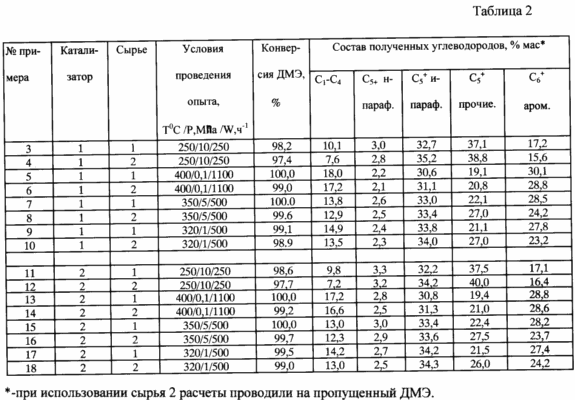
In the presented embodiments of the method for producing liquid hydrocarbons from dimethyl ether octane number (at IM) liquids not lower than 92. The obtained aromatic gasoline fraction does not exceed 30 wt.%, And they can be used as a basis for producing high-octane gasoline with a low content of aromatic hydrocarbons. High octane hydrocarbons produced is achieved not only due to the aromatic hydrocarbons, but also due to a higher (than in the prior art) and iso-paraffin content tsiklostroeniya.
CLAIM
1. A catalyst for producing liquid hydrocarbons from dimethyl ether a crystalline pentasil-type aluminosilicate with an SiO 2 / Al 2 O 3 = 25 - 100, containing 0.05 -. 0.1% by weight sodium oxide, and a binder component, wherein further comprising zinc oxide and oxides of rare earth elements in the following ratio, wt.%:
- ZnO - 0,5 - 3,0
- REE oxides - 0.1 - 5.0
- The crystalline aluminosilicate - 65 - 70
- Binder - The rest
2. A process for producing liquid hydrocarbon from dimethyl ether in the presence of a catalyst based on a crystalline pentasil-type aluminosilicate with an SiO 2 / Al 2 O 3 = 25 - 100, containing 0.05 -. 0.1% by weight sodium oxide, and a binder component, wherein in that the catalyst additionally contains zinc oxide and oxides of rare earth elements in the following ratio, wt.%:
- ZnO - 0,5 - 3,0
- REE oxides - 0.1 - 5.0
- The crystalline aluminosilicate - 65 - 70
- Binder - The rest
and preliminarily subjected to activation in air at 540 - 560 o C.
3. A method according to claim 2, characterized in that the contact with the catalyst of dimethyl ether is carried out at a volumetric feed rate of dimethyl ether 250 -. 1100 h -1 (gas) pressure of 0.1 - 10 MPa and temperature of 250 - 400 o C.
print version
Publication date 07.04.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.