|
Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2235754
![]()
METHOD OF OXIDIZING MINERAL OXIDATION OF FOSSILS
WITH ULTRASOUND
The name of the inventor: EH Te Fu (US); MEY Hai (US); LU Steve Hung-Moe (US)
The name of the patentee: SALFKO, INC. (US)
Patent Attorney: Vysotskaya Nina Nikolaevna
Address for correspondence: 103735, Moscow, ul. Ilyinka, 5/2, OOO "Soyuzpatent", NNVysotskoy
Date of commencement of the patent: 2001.09.24
The invention relates to a method for desulfurizing petroleum and petroleum-based fuels. The method includes the steps of combining the fuel with an acidified aqueous solution containing water and hydroperoxide to produce a multiphase reaction medium, the pH of the aqueous solution corresponding to a pH of 1 to 30% by volume of an aqueous hydrogen peroxide solution. Ultrasound is applied to the multiphase reaction medium for a time sufficient to oxidize the sulfides in the fuel to the sulfones. The extraction of the sulfones is carried out in order to obtain an organic phase substantially free of sulphones. The method has a high efficiency in the removal of sulfur compounds from fuels.
DESCRIPTION OF THE INVENTION
The present invention relates to the field of desulfurization of petroleum and petroleum fuels.
Although alternative energy sources are being developed and used in many countries, fossil fuels remain the largest and most frequently used source of energy due to their high efficiency, reliable characteristics and relatively low prices. Fossil fuels take various forms that vary from petroleum fractions to coal, bituminous sands and bituminous shales, and their applications range from consumer use, such as in automotive engines and for heating houses, to industrial applications such as in boilers, ovens, Melting furnaces and power plants.
The constant problem in the processing and use of fossil fuels is the presence of sulfur, mainly in the form of organic sulfur compounds. Sulfur takes part in the corrosion of pipelines, pumping and oil refineries and premature damage to internal combustion engines. Sulfur and is responsible for the poisoning of catalysts used in the processing and combustion of fossil fuels. As a result of poisoning of catalytic blocks in automobile engines, sulfur is partly responsible for emissions of nitrogen oxides (NO x ) from trucks and buses with a diesel engine. Sulfur and is responsible for the release of soot particles from trucks and buses, as the traps used in such vehicles to reduce particulate emissions are rapidly destroyed by using fuels with a high sulfur content. Apparently, the most well-known characteristic of sulfur compounds in fossil fuels is the conversion of these compounds to sulfur dioxide in the combustion of fuels. Release of sulfur dioxide into the atmosphere leads to acid rains, precipitation of acid, which has a harmful effect on agriculture, wildlife and human health. Shocked and subject to irreversible changes in the ecosystem of different types, and quality of life.
In connection with these problems, the Clean Air Act (1964) was adopted and various amendments, including the 1990 and 1999 amendments, which consistently imposed increasingly stringent requirements, in order to further reduce the amount of sulfur released into the atmosphere.
A recent US Environmental Protection Agency (EPA) document has reduced the standard sulfur content in diesel from 500 ppmw (ppmw) currently to 15 ppmw by mid 2006. For the reformulated Gasoline the norm according to the existing standard 300 ppmw was reduced to 30 pbw ppm by January 1, 2004, when the document comes into force. Similar changes in the laws have been adopted in the European Union, in which a sulfur content limit of 50 ppmw will be introduced for both gasoline and diesel fuel in 2005.
In connection with these Legislative Acts, there is always a need for more efficient methods of desulfurization. In addition to the difficulties of reducing sulfur emissions in accordance with the requirements, the refining industry was faced with the problem of increasing production costs associated with laborious methods of desulfurization and the adverse reaction of consumers and the government to higher prices. The costs associated with fossil fuels are some of the main factors affecting the world economy.
The most common method for desulfurizing fossil fuels is hydrodesulfurization, in which fossil fuels interact with hydrogen gas at elevated temperature and high pressure in the presence of an expensive catalyst. In this method, the organic sulfur is reduced to gaseous hydrogen sulfide, which is then oxidized to elemental sulfur in the Claus process. However, unreacted hydrogen sulphide from this Claus process, even in small amounts, is harmful. Hydrogen sulphide has an extremely high toxicity, which has led to many deaths in the workplace and in areas of natural accumulation, and is dangerous for workers. These hazards pose a health risk to people in many industries, such as gas, oil, chemical, geothermal, mining, drilling and metal smelting. Even short-term exposure to hydrogen sulfide at a concentration of 140 mg / m 3 causes conjunctivitis and keratitis, whereas exposure to 280 mg / m 3 of hydrogen sulfide (and above) can lead to loss of consciousness, paralysis and even death. The influence of hydrogen sulphide leads to disorders of the nervous system and is associated with cardiovascular, gastrointestinal and eye diseases. One of the problems with the new sulfur regulations is that when performing desulfurization under more stringent conditions that are required to achieve a lower sulfur content, there is an increased risk of hydrogen leakage through the reactor walls.
In addition to the tendency to release hydrogen sulphide into the atmosphere, the desulfurization process has some limitations on the ability to convert a variety of organosulfur compounds that are present in fossil fuels. Among these sulfur compounds, mercaptans, thioethers and disulphides are relatively easily removed during desulfurization. However, other sulfurous organic compounds are not easily removed, and more stringent reaction conditions are required. Such compounds include aromatic compounds, cyclic compounds and fused polycyclic compounds. Examples of these compounds are thiophene, benzothiophene, dibenzothiophene, other thiophenes with fused rings and various substituted analogues of these compounds. These compounds, which contain up to 40% of the total sulfur content in the Middle East crude oil and up to 70% of the sulfur content in East Texas crude oil, are the most difficult to remove and, for this reason, are in the center of attention in desulfurization research. The reaction conditions necessary to remove such compounds are so stringent that the decomposition of the fuel itself occurs, which leads to a decrease in its quality.
In this invention, it has been found that organic sulfur compounds can be removed from fossil fuels (or petroleum origin) by a method in which oxidative desulfurization is combined using ultrasound. Oxidative desulfurization is achieved by combining fossil fuels with a hydroperoxide oxidizing agent in the presence of an aqueous liquid, and the resulting mixture is sonicated to enhance the reactivity of the particles in the mixture. The unusually high efficiency of the process is evidenced by the fact that dibenzothiophene and related sulfur-containing organic sulfides, which are the most persistent organic sulfur compounds in fossil fuels, are easily converted by this method into the corresponding sulfones under relatively mild conditions of temperature and pressure. The increased polarity of the sulfones compared to sulfides provides a high degree of susceptibility to removal using traditional polar separation processes. Thus, dibenzothiophenes and other sulfides with comparable or less oxidation resistance in this process can be converted to more polar sulfone analogs, without external application of heat or pressure, otherwise under conditions that are created inside, in a highly localized area of the mixture, under the action of Ultrasound.
An advantage of the process according to the invention is that the oxidation proceeds selectively with respect to the conversion of sulfur-containing compounds, and no changes are observed in the sulfur-free fossil fuel components. In addition, although the aqueous as well as the organic phase remain in the form of an emulsion present during the conversion, this process can be carried out with a beneficial effect, without adding surfactants. Although the authors do not intend to associate themselves with any particular theory, it is assumed that most fossil fuels contain native (i.e., naturally present) compounds that play the role of surfactants. Another advantage is that the conversion takes place in a very short time, that is, significantly less than one hour, preferably less than 20 minutes, and in many cases less than 10 minutes.
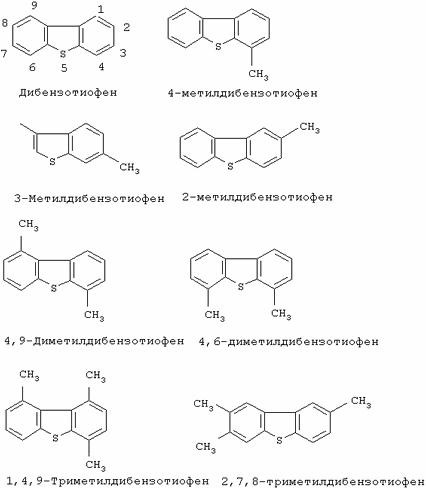
DETAILED DESCRIPTION OF THE INVENTION AND THEIR SPECIFIC EMBODIMENTS Organic sulfur that is present as natural components of fossil fuels (or derived from petroleum) includes a wide variety of compounds that are mainly hydrocarbons containing one or more sulfur atoms covalently bound to the remainder Part of the molecular structure. There are many compounds of oil origin containing carbon, hydrogen and sulfur atoms, some of these compounds containing other heteroatoms. The hydrocarbon moieties of these compounds may be aliphatic, aromatic, saturated, unsaturated, cyclic, fused cyclic or other nature, and sulfur atoms may be included in the molecular structure in the form of thiols, thioethers, sulfides, disulphides, and the like. Some of these most stable compounds are heterocycles containing sulfur, both aromatic and nonaromatic, this range starts from thiophene to condensed structures such as substituted and unsubstituted benzothiophenes and substituted and unsubstituted dibenzothiophenes. The structures of some of these compounds (and the numbering order used by the nomenclature) are shown below.
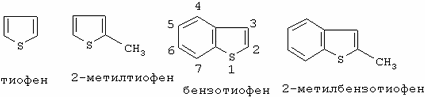
|
Other examples are analogs in which the methyl groups are replaced by ethyl or other lower alkyl or alkoxy groups or substituted alkyl groups, such as hydroxyl-substituted groups. The term "hydroperoxide" is used in this invention to designate a compound of the molecular structure of ROOH in which R is either a hydrogen atom or an organic or inorganic group. Examples of hydroperoxides in which R is an organic group are water-soluble hydroperoxides such as methyl hydroperoxide, ethyl hydroperoxide, isopropyl hydroperoxide, n-butyl hydroperoxide, sec-butyl hydroperoxide, tert-butyl hydroperoxide, 2-methoxy-2-propyl hydroperoxide, t-amyl hydroperoxide, and Cyclohexyl hydroperoxide. Examples of hydroperoxides in which R is an inorganic group are peroxo-azoic acid, peroxophosphoric acid and peroxosulfuric acid. Preferred hydroperoxides are hydrogen peroxide (in which R is a hydrogen atom) and tert-alkylperoxides, especially tert-butyl peroxide. The aqueous liquid that is combined with fossil fuel and hydroperoxide may be water or any aqueous solution. The relative amounts of liquid fossil fuel and water may vary, and although this may affect the efficiency of the process or the ease of handling liquids, these relative amounts are not essential for the present invention. However, in most cases, the best results will be achieved when the volume ratio of the fossil fuel to the aqueous liquid is about 1: 1 to 1: 3, and preferably about 1: 1.5 to 1: 2.5. |
The amount of hydroperoxide relative to the fossil fuel and the aqueous liquid, and may vary, and although the conversion may vary to some extent depending on the proportion of hydroperoxide, in fact this fraction is not significant for the present invention, any excess amounts of hydroperoxide can be removed by ultrasound. When the hydroperoxide is hydrogen peroxide (H 2 O 2 ), usually the best results will be achieved for most systems at a concentration of H 2 O 2 in the range of from about 1% to about 30% by volume (as H 2 O 2 ) of the combined aqueous and organic And preferably from about 2% to about 4%. For hydroperoxides other than H 2 O 2 , the preferred relative volumes may be equivalent molar quantities.
According to this invention, sound energy is applied by using ultrasound, which is waves similar to sound, whose frequency exceeds the frequency range for normal human hearing, i.e., above 20 kHz (20,000 oscillations per second). Ultrasound energy is generated at frequencies up to 10 GHz (10 10 vibrations per second), but for the purposes of this invention, effective results will be achieved for frequencies in the range of about 20 kHz to 200 kHz, and preferably within an interval of about 20 to 50 kHz. Ultrasonic waves can be generated from a source of mechanical, electrical, electromagnetic, or thermal energy. The intensity of sound energy and can vary within wide limits. For the purposes of this invention, effective results will be achieved with an intensity in the range of from about 30 W / cm 2 to 300 W / cm 2 or preferably from about 50 W / cm 2 to 100 W / cm 2 . A typical electromagnetic energy source is a magnetostrictive transducer that converts electromagnetic energy into ultrasonic energy using a highly variable magnetic field that acts on certain metals, alloys, and ferrites. A typical electrical power source is a piezoelectric transducer that uses natural or synthetic single crystals (such as quartz) or ceramic materials (such as barium titanate or lead zirconate) and which are acted upon by an alternating electric field across the opposite faces of the crystal or ceramic, in order To cause alternating expansion and contraction of a crystal or ceramic in the region of operating frequencies. Ultrasound is widely used in such areas as cleaning products in the electronic, automotive, aviation industry and with accurate measurements in the industry for measuring the flow rate in closed systems, such as the cooling agent system at nuclear power plants or the blood flow velocity in the vascular system, Materials, in mechanical processing, soldering, and welding, in electronics, in agriculture, oceanography and for the image of organs in medicine. Various methods for the production and application of ultrasonic energy and industrial suppliers of ultrasonic equipment are well known to those skilled in the art.
The duration of the action of ultrasound on the reaction system according to the invention is not significant in the practical successful implementation of the invention, the optimum duration being variable in accordance with the type of fuel being processed. However, an advantage of the invention is that effective and useful results can be achieved when exposed to sound energy for a relatively short time, specifically less than 20 minutes and in many cases less than 10 minutes. Sound energy can affect the entire system in a closed reaction space or flow system, while the exposure time is the residence time of the reaction mixture in a flowing ultrasonic chamber.
Although the authors do not intend to associate themselves with any particular theory, it is known that when ultrasound is applied to a liquid-phase system, cavitation occurs in the liquid, that is, continuous formation and disappearance of microscopic vacuum zones with very high local values of temperature and pressure. For example, it is believed that ultrasonic waves with a frequency of 45 kHz result in the formation of 90,000 sequences of explosions directed inward in 1 second, while local temperatures and pressures are approximately 5000 ° C and 31.4 MPa (4500 psi), respectively . This leads to very high turbulence and intensive mixing.
In certain embodiments of this invention, the process is carried out in the presence of a phase transfer agent. A large number of phase transfer agents are known which are effective for increasing the rate of reaction in systems that contain both an aqueous and an organic phase, many of which can be used to achieve the beneficial effect of the present invention. Cationic, anionic and non-ionic surfactants can act as phase transfer agents. Preferred phase transfer agents are cationic species and the preferred ones are quaternary ammonium salts, quaternary phosphonium salts and crown ethers. Examples of quaternary ammonium salts are tetrabutylammonium bromide, tetrabutylammonium hydrogen sulfate, tributylmethylammonium chloride, benzyltrimethylammonium chloride, benzyltriethylammonium chloride, methyltricaprilammonium chloride, dodecyltrimethylammonium bromide, tetraoctylammonium bromide, cetyltrimethylammonium chloride, and trimethyloctadecylammonium hydroxide. Particularly preferred are quaternary ammonium halides and most preferred are dodecyltrimethylammonium bromide and tetraoctylammonium bromide. An effective amount of the phase transfer agent can be any amount that results in an increase in the rate at which the sulfides are converted to sulfones, to increase the yield or selectivity of the process. In most cases, this effective amount can vary from about 0.2 g of the agent per liter of the reaction medium to 50 g / l of the agent and preferably from about 0.3 g / l to 3 g / l of the agent.
In additional embodiments of the invention, a metal catalyst is introduced into the reaction system to control the activity of the hydroxyl radical formed from the hydroperoxide. Examples of such catalysts are Fenton (ferrous salt) catalysts and metal ion catalysts, such as iron (II), iron (III), copper (I), copper (II), chromium (III), chromium (VI) , Ions of molybdenum, tungsten and vanadium. Among these catalysts, iron (II), iron (III), copper (II) and tungsten catalysts are preferred. For some systems, such as crude oil, Fenton-type catalysts are preferred, although for other systems, such as diesel fuel, in which dibenzothiophene is the main sulfur component, tungstates are preferred. Tungstates include tungstic acid, substituted tungstic acids, such as phosphotungstic acid and metal tungstates. When a metal catalyst is present, it is used in a catalytically effective amount, this means any amount that will intensify the process towards the desired goal, i.e. the oxidation of the sulfides to the sulfones. In most cases, the catalytically effective amount will vary from about 1 to 300 mmol / L and preferably from about 10 to 100 mmol / L.
In the process of oxidation, ultrasound generates heat, and the addition of heat from external sources is not required. To maintain control over the course of the process, heat is preferably removed from the reaction mixture using a refrigerant or cooling device or means. When cooling is achieved by immersion of the ultrasonic chamber in the refrigerant bath, its temperature can be about 50 ° C or lower, preferably about 20 ° C or lower, and more preferably in the range of about -5 ° C to 20 ° C. Suitable cooling methods or Devices are quite obvious to those skilled in the art.
When the ultrasound action stops, the resulting mixture will contain an aqueous and organic phase, the organic phase containing the mass of the sulfones produced during the oxidation process. The product mixture can be subjected to phase separation prior to the removal of the sulfone, or it is possible to separate the phases from the multiphase mixture, without separating the phases. If desired, the phase separation can be carried out by conventional methods, if necessary, this may be preceded by the destruction of the emulsion formed by ultrasound. Destruction of emulsion and is carried out by traditional means. Various possibilities for the implementation of these techniques can readily be understood by those skilled in the field of emulsions, and especially with oil-in-water emulsions.
As a result of the increased polarity of the sulfones compared to the starting sulfides present in fossil fuels, the sulfones obtained according to the invention can be easily removed from either the aqueous and organic phases or from both phases by conventional methods for extracting polar molecules. The sulfones can be extracted by solid-liquid extraction, using absorbers such as silica gel, activated alumina, polymeric resins and zeolites. Alternatively, the sulfones can be extracted by liquid-liquid extraction, using polar solvents such as dimethylformamide, N-methylpyrrolidone, or acetonitrile. Other extraction media, both solid and liquid, will be readily apparent to specialists in the field of polar substances.
The term "liquid fossil fuel" is used herein to refer to any carbonaceous liquid that comes from oil, coal or any other material of natural origin and which is used to produce energy of any type of use, including industrial, commercial, governmental and domestic use. These types of fuel include automotive fuel, such as gasoline, diesel fuel, jet fuel and rocket fuel, as well as fuel oil based on oil residues, including fuel oil and residual fuel. Fleet fuel oil is a heavy residual hydrocarbon that is used as fuel in the fleet and in industry, as well as in large-scale heating installations. Boiler fuel No. 6, which is known as "bunker fuel C", is used in power plants operating on fuel oil as the main fuel, and is used as the main fuel for power plants in ships of deep diving in the fleet. Boiler fuel brands Nos. 4 and 5 are used to heat large buildings, such as schools, residential houses and company buildings, and for large stationary marine engines. The heaviest boiler fuel is a vacuum residue from fractional distillation, commonly referred to as a "vacuum residue" having a boiling point of 5b5 ° C or higher that is used as asphalt and coker feed. The present invention is applicable to reducing the sulfur content of any of these fuels and boiler fuel.
Since the reaction medium in which oxidative desulfurization according to the invention is carried out is an emulsion, the invention is particularly applicable to the preparation of emulsion fuels. Examples of such fuels are disclosed in US Pat. No. 5,156,114, issued October 20, 1992 to RW Gunnerman, issued May 14, Replacement Patent Re 35237, and copending US Patent Application Serial No. 09/081867, filed May 20, 1998. The description of these Patents and this patent application under consideration is included in the present invention as a reference for all legal purposes capable of facilitating the consideration of this application. The emulsion fuel contains an oil-in-water emulsion and can be obtained directly from the ultrasonic-treated reaction mixture and the extraction of sulfones, by adding additives stabilizing the emulsion.
The following examples are provided for purposes of illustration and are not intended to limit the scope of the invention.
EXAMPLE 1
This example illustrates the use of the process of the present invention for removing dibenzothiophene from a solution of dibenzothiophene in toluene and from crude oil and demonstrating the effect of changing certain parameters of the reaction system. The following instruments and materials are used:
- Ultrasound generator:
Supplier: Sonics & Materials, Inc., Newtown Connecticut, USA
Model: VCX-600
Energy supply: the total output energy is 600 W
Frequency: 20 kHz
- Transducer type: piezoelectric PZT; Crystals of lead zirconate titanate
- Type of probe: ultrasonic probe with a threaded head 1/2 inches (12.7 mm)
- Intensity: up to 100 W / cm 2
- Sulfur Analyzer:
Supplier: Horiba Instruments, Inc., Knoxville, Tennessee, USA
Model: SLFA-20
Detection limit: 20 ppm
- Gas chromatograph: Hewlett Packard 5880A
- UV / Visible Spectrophotometer: Hewlett Packard 8452A
- Hydroperoxide: 30% H 2 O 2 by weight (wt.%) In water
- Dibenzothiophene (DBT) in toluene: initial sulfur content of 0.38% by weight as elemental sulfur
- Crude oil: oil from Fancher Oil Co. From Wyoming; Initial sulfur content 3.33% by weight
A solution of dibenzothiophene in toluene was combined with an aqueous solution of H 2 O 2 and a phase transfer agent, a quaternary ammonium salt and phosphotungstic acid, was added. The mixture is sonicated for 20 minutes and after extraction of the acetonitrile product mixture the following result is obtained: the sulfur content is reduced from the starting level of 0.38% to the final level of 0.15% by weight, that is, the degree of sulfur removal is 60.5%. When the UV spectrum of the solution was compared to the reaction with the product solution spectrum, it was found that both peaks observed in the original spectrum disappeared in the product spectrum: this indicates that the oxidation process leads to significant changes in the structure of dibenzothiophene in the sample. Analysis of the solutions before and after the reaction on the gas chromatograph shows that for the peak associated with the phase transfer agent, the changes are very small or absent, while the intensity of the peak related to dibenzothiophene in the product is too low for detection: there are only traces of dibenzothiophene After concentration of the product mixture. These results indicate a high efficiency and selectivity of the process with respect to the oxidation of dibenzothiophene.
In crude oil tests, the total volume of the sample is 90 ml, and the oil / water volume ratio is 4: 5. These tests are carried out without adding a phase transfer agent at ultrasound intensity of 60%. In the first series of tests, only oil and water are sonicated (without the addition of a hydroperoxide, a phase transfer agent or a Fenton catalyst), and the sonication time varies between 2 minutes and 10 minutes. Table 1, below, shows the sulfur content of the sample relative to the original content (before any treatment).
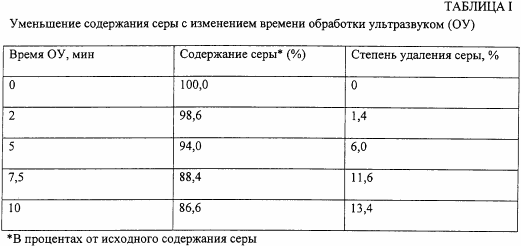
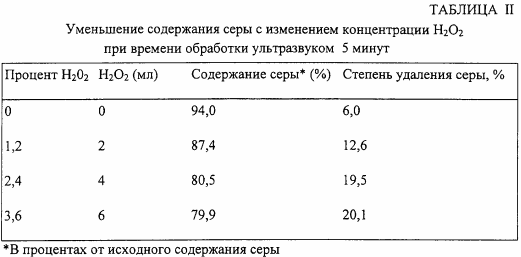
In the second series of tests, hydrogen peroxide is added to the reaction mixture at a concentration ranging from 1.2% to 6%, with ultrasound processing time being limited to 5 minutes. The results are shown in Table II.
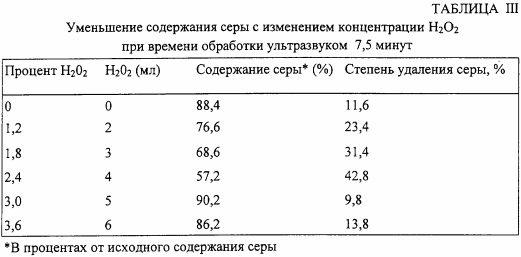
In the third test series, various amounts of H 2 O 2 are added to the reaction mixture, and the sonication time is increased to 7.5 minutes. The results are shown in Table III.
In the fourth series of tests, the Fenton catalyst, FeSO 4 , is added to the reaction mixture, and the amount of HsO3 is varied (the sonication time is 7.5 minutes). The results are shown in Table IV.
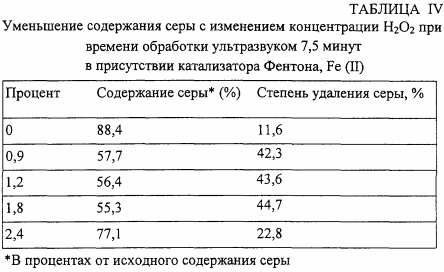
In the fifth series of tests, Fenton catalysts of various types are used, at the same concentration (40 mmol / L), with the addition of 2.4% H 2 O 2 (4 ml), with a sonication time of 5 minutes. The results are shown in Table V.

EXAMPLE 2
This example illustrates the effect of additional variations in the method of the invention, including the use of various metal catalysts and variations in the oil / water ratio, ultrasound intensity, temperature, sonication time, amount of H 2 O 2 , and catalyst selection. The materials and instruments are the same as in Example 1.
A toluene solution of dibenzothiophene, with H 2 O 2 and a quaternary ammonium salt is used at a sonication time of 7 minutes. Three types of catalysts are tested: tungstate (phosphorvanidic acid), molybdate and Fe (II). The degree of sulfur removal by the tungstate catalyst is 74.6%, while the degree of sulfur removal by both molybdate and Fe (II) catalysts was lower than 5%. Additional tests are then carried out using different amounts of the tungstate catalyst. With a total volume of the reaction mixture of 90 ml, 0.6 g of phosphorvanidic acid provides a degree of sulfur removal of 51.2%, 1.2 g of catalyst provide a degree of sulfur removal of 74.6%, and 2.5 g of catalyst remove 70.1% of sulfur. The product was analyzed by IR spectroscopy using a Fourier spectrophotometer system, the 5-DX-FTIR model (from Nicolet Inc.) with a Hewlett Packard 7475A plotter. In accordance with standard IR spectra, the sulfonic group has two strong bands near 1135 cm -1 (asymmetric, stretched) and 1300 cm -1 (symmetrical, stretched), respectively. Both these bands are observed in the product spectra. This indicates that the solid product, of course, is a sulphone of dibenzothiophene.
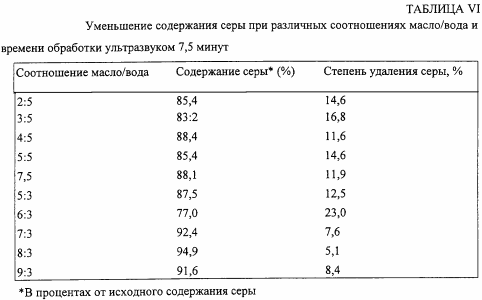
Samples of high-sulfur crude are then subjected to several tests using distilled water. In the first series of these experiments, the oil / water ratio varies, while in each test the sonication time is 7.5 minutes, the temperature being increased to 90 ![]() C. The results are shown in Table VI.
C. The results are shown in Table VI.
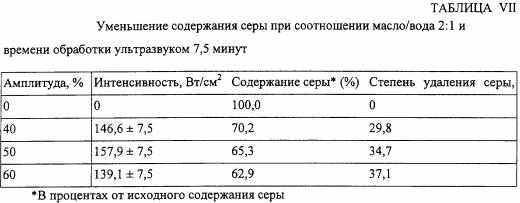
In a second series of experiments, the ultrasound intensity is varied using an oil / water volume ratio of 2: 1 and a sonication time of 7.5 minutes, the ultrasonic chamber being immersed in ice-cooled water. The results are shown in Table VII.
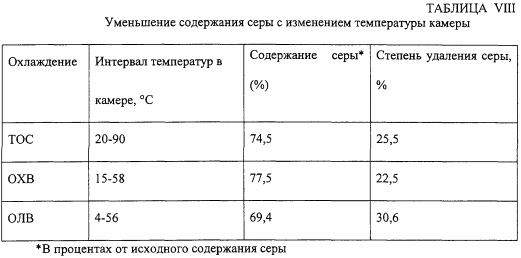
In the third series of experiments, the temperature is varied using an oil / water ratio of 2: 1, an ultrasonic treatment time of 7.5 minutes, and an ultrasound amplitude of 50% (157.9 ± 7.5 W / cm 2 ). The results are shown in Table VIII. One test is carried out at ambient temperature, without a cooling system (denoted in Table "TOC"), a second experiment is carried out by immersing the ultrasonic chamber in a cold water bath (designated in the "OXB" table) and a third experiment - immersing the ultrasonic chamber in water , Cooled by ice (designation in the table "OLV").
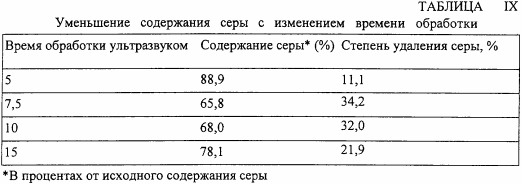
In the fourth series of experiments, the processing time is varied by ultrasound using an ice-water cooling system and other conditions identical to those in the third series of experiments. The results are shown in Table IX.
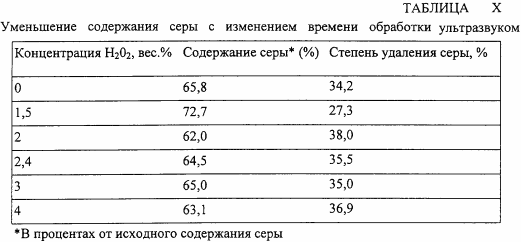
In the fifth test series, the concentration of H 2 O 2 is varied using the sonication time of 7.5 min and other conditions identical to those in the fourth series of runs. The results are shown in Table X.
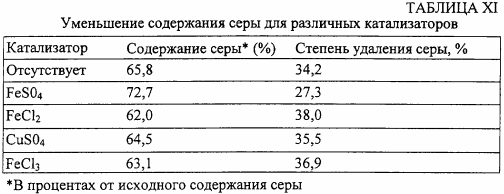
In the sixth series of tests, other metal catalysts are used at a concentration (40 mmol / L) supplemented with 2% H 2 O 2 and other conditions identical to those in the fifth series of runs. The results are shown in Table XI.
EXAMPLE 3
This example illustrates the effect of the process according to the invention on three different sulfur compounds: dibenzothiophene (DBT), benzothiophene (BT), and thiophene. Each sulphide compound is tested in a solution of toluene with an elemental sulfur content of 0.4% by weight. In each case, 20 g of solution are added to the reaction vessel, with addition of 0.12 g of phosphotungstic acid, 0.1 g of tetraoctylammonium bromide and 40 g of a 30% (by volume) solution of H 2 O 2 in water. The mixture is sonicated at a frequency of 20 kHz, at an intensity of 50%, for 7 minutes using a refrigerant at a temperature of 20 ° C and 4 ° C. The materials and instruments are the same as those in the preceding Examples. The results in percent removal are shown in Table XII.
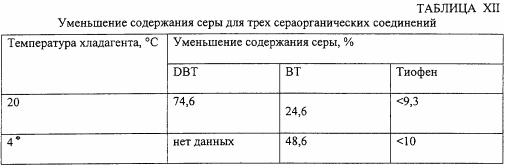
This experiment was then repeated for DBT except that gasoline (with a sulfur content of 20 ppm), instead of toluene, was used as the solvent. At a coolant temperature of 20 ° C, the sulfur removal rate is 98.4%, and at 4 ° C sulfur is removed by 99.2%.
EXAMPLE 4
This example illustrates the effect of various process variables on the degree of sulfur removal in crude oil in the process according to the invention. Five process parameters are varied, each on two levels (see Table XIII).
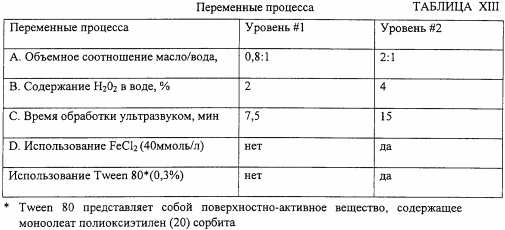
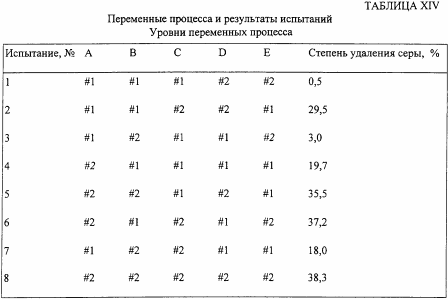
Then eight tests are carried out using various combinations of these process variables at an ultrasound amplitude of 50%, and an ice water cooling system. The degree of sulfur removal in percent is determined in each test, and the results are shown in Table XIV.
EXAMPLE 5
This example illustrates the use of two different hydroperoxides, H 2 O 2 and tert-butyl hydroperoxide in the process of the invention. The process is carried out on heavy crude oil, in all other uses the materials and devices specified in the preceding examples. Process parameters are listed below:
- Volumetric ratio oil
- Total volume of oil / water mixture: 90 ml / water: 2: 1
- Temperature control: by immersion in a bath with ice-cold water
- Amplitude of ultrasound: 50%
- Processing time by ultrasound: 7.5 min
- Average intensity of ultrasound: 111 W / cm 2
- The concentration of hydroperoxide (both H 2 O 2 and tert-butyl hydroperoxide): 2% by volume in water.
For each hydroperoxide, the degree of sulfur removal is determined and the results are shown in Table XV below.

EXAMPLE 6
This example illustrates the effect of various surfactants or phase transfer agents on the effectiveness of the process of the invention. The process is carried out in a toluene solution of dibenzothiophene, and materials and instruments use the same as in the preceding examples, along with the optimum conditions specified for these examples. The following surfactants are used:
- Dodecyltrimethylammonium bromide (DOB)
- Bromide tetraoctylammonium (TEB)
- 1-octanesulfonic acid sodium salt
- Span 20 (monolauryl ether of sorbitol) Tween 80 (polyoxyethylene (20) sorbitan monooleate).
Of these surfactants, only DOB and TEB accelerate the desulfurization process.
EXAMPLE 7
This example illustrates the use of the process according to the invention for desulfurizing diesel fuel. High-sulfur and low-sulfur diesel fuel were investigated, the first fuel having an initial sulfur content of 0.1187 wt .-%, and the second fuel having an initial sulfur content of 0.0190%.
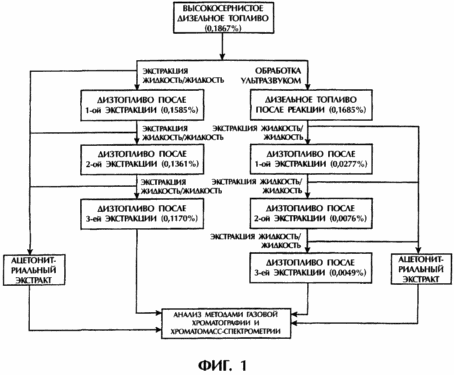
FIG. 1 shows a schematic diagram of the process used for high sulfur diesel fuel, comparing the results obtained using ultrasound to the results obtained without using ultrasound. The term "L / L Extraction" means liquid-liquid extraction using acetonitrile as the extracting solvent, in each case 3 extractions are carried out. The left side of the circuit shows the comparative method, without using ultrasound, and as a result of these three extractions the sulfur content is obtained, respectively: 0.1585%, 0.1361% and 0.1170%. On the right, the results of the same method performed with ultrasound are shown, as a result of these three extractions, the sulfur content is obtained, respectively: 0.02277%, 0.0076% and 0.0049% (final sulfur removal 97.4%).
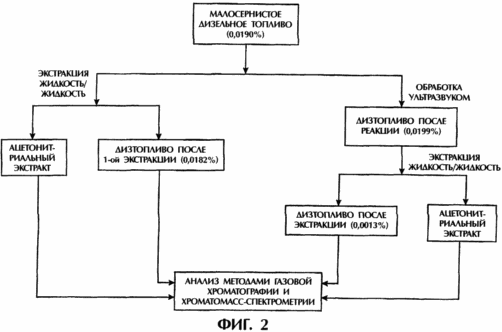
FIG. 2 shows a schematic diagram of the process used for low-sulfur diesel fuel, comparing the results obtained using ultrasound to the results obtained without using ultrasound. The term "L / L Extraction" means liquid-liquid extraction using acetonitrile as an extracting solvent, in each case only one extraction is carried out. The left side of the diagram shows a comparative method without the use of ultrasound, which results in the extraction of sulfur content 0.0182% after extraction. On the right, the results of the same method performed with ultrasound are shown, as a result of which, after extraction, the sulfur content is obtained, respectively: 0.0013% (final sulfur removal 93.2%).
 |
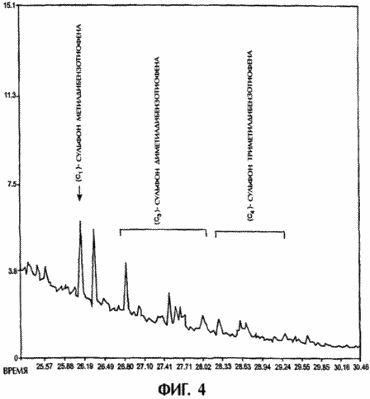 |
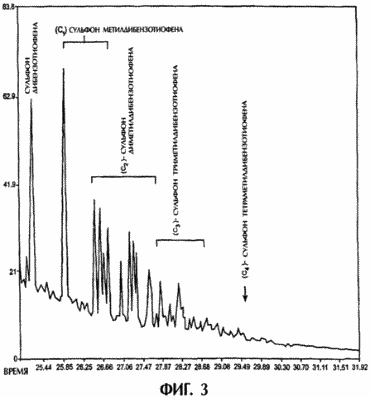
FIGS. 3 and 4 show the scanning cycles of chromatography-mass spectrometric analysis of high-sulfur diesel fuel and low-sulfur diesel fuel, respectively. Each fuel is combined with the corresponding acetonitrile extracts obtained in the processes shown in FIGS. 1 and 2. Each scan cycle represents only samples processed with ultrasound, and it can be seen on the scans that dibenzothiophene and most of the alkyl-substituted dibenzothiophenes in both diesel fuel samples are converted In the corresponding sulfones.
The foregoing description is intended primarily to illustrate the invention. Those skilled in the art will readily recognize additional variations in materials, additives, operating conditions and equipment that are all within the scope of the present invention.
CLAIM
1. A method for removing sulphides from liquid fossil fuels, characterized in that it comprises the following steps: a) combining said fossil fuel with an acidified aqueous solution containing water and hydroperoxide to produce a multiphase reaction medium, the pH of the aqueous solution corresponding to the pH of 1- 30 vol.% Aqueous hydrogen peroxide solution; B) the effect of ultrasound on the multiphase reaction medium for a time sufficient to oxidize the sulphides in the fossil fuel to the sulfones; And c) extracting the sulfones to obtain an organic phase substantially free of sulphones.
2. The process of claim 1, wherein the hydroperoxide is selected from the group consisting of hydrogen peroxide and water-soluble alkyl hydroperoxides.
3. The process of claim 1, wherein the hydroperoxide is selected from the group consisting of hydrogen peroxide and a tertiary alkyl hydroperoxide.
4. The process of claim 1, wherein the hydroperoxide is selected from the group consisting of hydrogen peroxide and tertiary butyl hydroperoxide.
5. The process of claim 1, wherein the hydroperoxide is hydrogen peroxide.
6. The method of claim 1, further comprising combining a phase transfer agent with liquid fossil fuel and an acidified aqueous solution to produce a multiphase reaction medium.
7. The method of claim 6, wherein the phase transfer agent is a cationic agent.
8. The process of claim 7, wherein the cationic phase transfer agent is a quaternary ammonium salt.
9. The process of claim 8, wherein the quaternary ammonium salt is a tetraalkylammonium halide.
10. The method of claim 1, wherein the liquid fossil fuel and the acidified aqueous solution in step a) are taken in a volume ratio of about 1: 1 to 1: 3.
11. The method of claim 1, wherein the liquid fossil fuel and the acidified aqueous solution in step a) are taken in a volume ratio of about 1: 1.5 to 1: 2.5.
12. The method of claim 1, wherein step b) is carried out without heating the multiphase reaction medium from an external heat source.
13. The method of claim 1, wherein step b) is carried out by cooling the multiphase reaction medium by thermal contact with the cooling medium at a temperature of 50 ° C. or lower.
14. The method of claim 1, wherein step b) is carried out by cooling the multiphase reaction medium by thermal contact with the cooling medium at a temperature of 20 ° C. or lower.
15. The method of claim 1, wherein step b) is carried out by cooling the multiphase reaction medium by thermal contact with the cooling medium at a temperature of about -5 to 20 ° C.
16. The method of claim 1, wherein step b) comprises exposing the ultrasound to a frequency of approximately 20 to 200 kHz.
17. The method of claim 1, wherein step b) comprises applying ultrasound at a frequency of about 20 to 200 kHz, at an intensity of about 30 to 300 W / cm 2 .
18. The method of claim 1, wherein step b) comprises exposing the ultrasound to a frequency of about 20 to 50 kHz.
19. The method of claim 1, wherein step b) comprises exposing the ultrasound to a frequency of approximately 20 to 50 kHz at an intensity of about 50 to 100 W / cm 2 .
20. The process of claim 1, wherein step c) comprises i) separating the phases of the multiphase reaction medium into an organic and an aqueous phase, and ii) extracting said sulfones from the organic phase.
21. A method according to claim 20, characterized in that i) includes extraction of sulfones by liquid-liquid extraction with a polar solvent.
22. The method of claim 20, wherein i) comprises extracting the sulfones by solid-liquid extraction with silica gel.
23. The method of claim 1, further comprising combining a catalytic amount of a metal catalyst selected from the group consisting of iron (II), iron (III), copper (I), copper (II), chromium (III), chromium (VI), and molybdates, tungstates and vanadates, with said liquid fossil fuel and an acidified aqueous solution to form a multiphase reaction medium.
24. The method of claim 23, wherein the metal catalyst is selected from the group consisting of iron (II), iron (III), copper (II) and tungstate compounds.
25. The method of claim 23, wherein the metal catalyst is tungstate.
26. The method of claim 1, wherein the liquid fossil fuel is selected from the group consisting of crude oil, bituminous shale, diesel fuel, gasoline, kerosene, liquefied petroleum gas and oil-based boiler fuels.
27. The method of claim 1, wherein the liquid fossil fuel is selected from the group consisting of diesel fuel, gasoline, kerosene, and oil-based boiler fuels.
28. The method of claim 1, wherein the liquid fossil fuel is a crude oil.
29. The method of claim 1, wherein the liquid fossil fuel is diesel fuel.
30. The method of claim 1, wherein the liquid fossil fuel is boiler fuel No. 6 (bunker fuel C).
31. The method of claim 1, wherein the liquid fossil fuel is a vacuum residue from the distillation of oil.
32. The method of claim 1, wherein the time of step b) is less than 20 minutes.
33. The method of claim 1, wherein the time of step b) is less than 10 minutes.
print version
Date of publication 07.04.2007gg




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.