| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2129587
![]()
Composition of liquid fuels and additive concentrates
Name of the inventor: Brian William Davis (GB); Kenneth Lyutas (GB); Alessandro Lombardi (GB)
The name of the patentee: Exxon Chemical, Inc. Peytents. (US)
Address for correspondence: 101000, Moscow, Small Zlatoustinskiy Lane 10-15, "EVROMARKPAT".
Starting date of the patent: 1993.10.21
The invention relates to compositions of liquid fuels, more particularly to compositions of liquid fuels that are susceptible to wax formation at low temperatures, described fuel oil composition based biofuel mixed with fuel oil, which consists in that it further comprises one or more additives that modify crystals paraffin and / or lowering the pour point of fuels selected from the group: oil-soluble ethylene copolymer, a comb polymer; polar nitrogen compound; a compound in which at least one essentially linear alkyl group having 10 - 30 carbon atoms attached to a non-polymeric organic residue to form at least one linear chain of atoms that includes the carbon atoms of said alkyl groups and one or more non-terminal oxygen atoms . Describes concentrate and additives. The technical result consists in the fact that the new compounds possess greater beneficial effect than the prior art.
DESCRIPTION OF THE INVENTION
The present invention relates to liquid fuel compositions, and more particularly to liquid fuel compositions that are susceptible to wax formation at low temperatures, and to additive compositions for such fuel oil compositions.
Fuel oils, whether derived from petroleum or from vegetable origin, contain components that at low temperature tend to precipitate as large crystals of wax in such a way that they form a gel structure which deprive the fuel flow properties. The lowest temperature at which the fuel will still flow is known as the pour point.
As fuel temperature decreases and approaches the pour point, difficulties arise in transporting the fuel through lines and pumps. Further, the wax crystals tend to plug fuel lines and filters at temperatures above the pour point. These problems are well known to those skilled in the art, so various additives have been proposed, many of which are in commercial use, for reducing the fuel oil pour point. Simultaneously, other additives have been proposed, used in the industry to reduce the crystal size and changing the shape of the wax crystals. Smaller size crystals are desirable since they this reduces the likelihood of filter clogging. Wax from a diesel fuel, which is primarily an alkane wax, crystallizes as platelets; certain additives inhibit this process, contributing to the fact that the wax to adopt an acicular habit, the resulting needles being more freely pass through a filter than are platelets. Additives may have the effect of retaining in suspension in the fuel the crystals, thereby reducing the deposition rate of the crystals, which helps to prevent clogging.
The fuels of vegetable origin, and known as biofuels, are believed to be less harmful when burning environment, and obtained from a renewable source. It has been reported that on combustion less carbon dioxide is formed than is formed by the equivalent quantity of petroleum distillate oil, such as diesel fuel, and forms a very small amount of sulfur dioxide. Certain derivatives of vegetable oils such as rapeseed oil, in particular obtained by saponification and re-esterification with a monohydric alcohol, may be used as a substitute for diesel fuel. It has recently been reported that mixtures of a rapeseed ester, for example, rapeseed oil methyl ester (RME), with petroleum distillate fuels in a relationship, such as 10: 90 by volume are likely to be commercially available in the near future.
Known fuel oil composition comprising a mixture of biofuels with petroleum-based fuels, as described in application FR 2492402, is selected as the closest analogue.
However, such mixtures may have poorer low temperature properties than their individual components. A measure of the flowability of fuels at low temperature is the temperature of the cold filter plugging (CFPP), the definition of which is described in "Journal of the Institite of Petroleum" 52 (1966) 173-185. In one case, described in more detail below, a mixture of equal volumes of a diesel fuel with a CFPP -6 o C, and an RME with a CFPP -13 o C, has only CFPP -5 o C, while a 90:10 mixture of diesel fuel: CFPP -4 RME has o C, both values being higher than the CFPP of either fuel alone.
A further problem encountered at temperatures low enough for wax formation in the fuel is paraffin deposition in the lower part of each storage tank. This phenomenon has two effects: the first is shown in the vessel itself where the settled layer of wax may block an outlet at the bottom, and the other - with the subsequent use of the fuel. The composition of the wax-rich portion of fuel will differ from the remainder, and will have poorer low temperature properties than the homogeneous fuel from which it is derived.
There are various additives available which change the nature of the wax formed, so that it remains in suspension in the fuel, achieving dispersion of waxy material throughout the depth of the container, with a greater or lesser degree of uniformity depending on the effectiveness of the additive on the fuel.
Known additive concentrate, lowering the pour point and / or modifying the wax crystals in the fuel oil as described in US Patent 4481013 is the closest analogue.
Although the mechanism of action of substances that lower the CFPP, and additives, wax anti-settling, is not fully understood, there is evidence that their effectiveness depends to a significant extent on matching of the alkanes in the fuel to alkyl or alkylene chains in the additive, the crystal growth of the alkane wax is performed, for example by co-crystallization of an alkyl chain of similar length in an additive.
Whereas the aliphatic middle distillate fuels contain largely alkanes, aliphatic moieties of biofuels contain a high proportion of unsaturated chains. For example, rapeseed oil typically contains the esters of, in addition to esters, ethers consisting of the sum of some C 16 -C 18 saturated fatty acid in an amount of 11-19%, 23-32% of certain mono-, di- and 40-50% 4-12% trinenasyschennyh C 18 -C 22 acids, primarily oleic, linoleic, linolenic and erucic acids. They do not crystallize the same way as do the saturated materials, and would therefore not be expected that the additives suitable for improving low temperature properties of petroleum fuels would be effective in biofuels, and that their effectiveness in mixtures of biofuels and petroleum fuels could be in the range corresponding proportion of petroleum fuel in the mixture.
The object of the present invention is to improve low temperature flow of biofuels with petroleum fuel blends.
The problem is solved composition based on liquid fuels with biofuels fuel oil mixture according to the invention which further comprises one or more additives modifying the wax crystals and / or lowering the pour point of fuels selected from the group: a) an oil-soluble ethylene copolymer; b) a comb polymer; c) a polar nitrogen compound; g) the compound in which at least one substantially linear alkyl group having 10-30 carbon atoms, attached to a non-polymeric organic residue to form at least one linear chain of atoms that includes the carbon atoms of said alkyl groups and one or more non-terminal oxygen atoms. Furthermore, the problem is solved concentrate additives that modify the wax crystals and / or reducing the fuel flow temperature based on the solvent, which according to the invention comprises additives as characterized above as a), b), c) and d), and the solvent comprises a biofuel or its mixture with fuel oil.
A preferred content of the additive in the fuel is 0,0005-1,0 wt.%.
As biofuel, or fuel derived from vegetable sources, particularly from an agricultural product may be used, for example, liquid fuel, especially an oil. A preferred oil is a vegetable oil, such as soybean, palm, sunflower, cottonseed, peanut, coconut or rapeseed oil, either by itself or, preferably, saponified and esterified (or interesterified), preferably a monohydric alcohol, particularly methanol. A preferred biofuel is rapeseed methyl ester.
Liquid fuel oil may be a distillate, especially a middle distillate, petroleum fraction. Such liquid fuels obtained by distillation, generally boil in the range from 100 o C to 500 o C, for example from 150 o C to 400 o C. The fuel oil may comprise atmospheric distillate or vacuum, or cracked gas oil or a mixture in any ratio directly cracked and thermal and / or catalytic cracked distillates. The most common liquid fuels obtained by distillation are kerosene, jet fuels, diesel fuels, heating oils and heavy fuel oils. Heating oil may be a straight atmospheric distillate, or it may contain vacuum gas oil or cracked components or both.
The invention is applicable to mixtures of fuels in all proportions; First of all, however, the composition comprises from 5 to 75%, more particularly from 10 to 50% of biofuel. Within the invention it is possible to use two or more petroleum fuels, or more specifically, two or more biofuels, in admixture with one or more other fuels.
Preferably, the mixture of the fuels of the invention contains less than 5 vol.% Of methanol, for example 4%, 3%, 2% or 1% or substantially does not contain methanol.
The components of the additive described in more detail below. It should be noted that individual polymers or compounds may fall within more than one of the definitions (a), (b), (c) and (d) set forth above.
(A) Oil soluble ethylene copolymers
The oil-soluble copolymer, component (a) may be a copolymer of ethylene with an ethylenically unsaturated ester, such as a copolymer of ethylene with an unsaturated carboxylic acid ester and saturated alcohol, but the ester is preferably an ester of an unsaturated alcohol with a saturated carboxylic acid. Copolymer of ethylene-vinyl ester is advantageous; preferred is ethylene-vinyl acetate copolymer, ethylene-vinyl propionate ethyl-vinyl hexanoate, or ethyl-viniloktanoat.
More specifically, the component (a) may comprise an ethylene copolymer having, in addition to units derived from ethylene, units of the formula
-CH 2- CRR 30-, X
wherein R represents H or CH 3 and R 30 is a group of the formula COOR 3 or OOCR 4, wherein R 3 and R 4 independently represent a hydrocarbyl group.
As described in US Patent 3961916, a composition comprising a substance that prevents the growth of wax crystals and the agent prevents nucleating additive is effective for improving fluidity at low temperatures for liquid fuels derived from crude oil distillation fractions medium. A substance that prevents the growth of, and an agent that prevents nucleation are preferably low molecular weight polymer based on ethylene-unsaturated ester with a higher ester content and a high molecular weight polymer based on ethylene-unsaturated ester with a lower ester content respectively. Advantageously both ether is vinyl acetate copolymers. It has been found that such a combination is extremely effective according to the present invention. More specifically, the combination comprising:
(I) an oil-soluble ethylene copolymer having, in addition to units derived from ethylene, from 7.5 to 35 mole percent of units of the formula
-CH 2 - CRR 1 I
(II) an oil-soluble ethylene copolymer having, in addition to units derived from ethylene, up to 10 mole percent of units of the formula
-CH 2 - CRR 2 II
wherein each R independently represents H or CH 2 and each R 1 and R 2 independently represents a group of the formula COOR 3 or OOCR 4, wherein R 3 and R 4 independently represent a hydrocarbyl group, the proportion of units 1 in the polymer (I) by at least 2 molar per cent greater than the proportion of units II in polymer (II).
The term "hydrocarbyl" as used herein refers to a group having a carbon atom directly attached to the remainder of the molecule and having a hydrocarbon or predominantly hydrocarbon character. Among such groups include hydrocarbon groups, including aliphatic (e.g. alkyl or alkenyl), alicyclic (e.g., cycloalkyl or cycloalkenyl), aromatic, aliphatic and alicyclic-substituted aromatic, and aromatic-substituted aliphatic and alicyclic groups. Aliphatic groups are advantageously saturated. These groups may contain non-hydrocarbon substituents provided their presence does not alter the predominantly hydrocarbon character. Examples include keto, halo, hydroxy, nitro, cyano, alkoxy and acyl groups. If the hydrocarbyl group is substituted, mono-substituent group is preferable. Examples of substituted hydrocarbyl groups include 2-hydroxyethyl, 3-hydroxypropyl, 4-hydroxybutyl, 2-ketopropyl, ethoxyethyl, and propoxypropyl. Groups may alternatively contain other than carbon atoms in a linear or cyclic structure, the rest consisting of carbon atoms. These heteroatoms can be, for example, nitrogen, sulfur, and preferably oxygen. Advantageously, the hydrocarbyl group contains at most 30 atoms, preferably not more than 15, more preferably not more than 10, still more preferably not more than 8 carbon atoms.
In formulas X, I and II, above, R preferably represents H and preferably R 3 and R 4 are independently an alkenyl or as indicated above, preferably the alkyl group, which is advantageously linear. If the alkyl or alkenyl group is branched, for example, a 2-ethylhexyl group, a carbon atom is advantageously part of a methylene group. The alkyl or alkenyl group preferably having up to 30 carbon atoms, preferably from 1 (2 in the case of alkenyl) to 14 carbon atoms, and more preferably from 1 to 10 carbon atoms. As the alkyl or alkenyl groups include: methyl, ethyl, propyl, n-butyl, isobutyl, and isomers, preferably the linear isomers, of pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl , octadecyl, nonadecyl and icosyl, and their corresponding alkenyl, advantageously alk-omega-enyl, radicals.
As cycloalkyl, alkaryl and aryl radicals include, for example, cyclohexyl, benzyl and phenyl.
The copolymer or copolymers may contain units of formulas other than those as mentioned above, for example units of the formula
-CH 2 - CRR 5 - III
where R 5 represents a -OH group, or of formula
-CCH 3 (CH 2 R 6) -CHR 7 - IV
where R 6 and R 7 each independently represents hydrogen or an alkyl group containing up to 6 carbon atoms, the moieties IV advantageously being derived from isobutylene, diisobutylene, 2-methylbut-2-ene or 2-methylpent-2-ene.
Units of the formula X, I or II may be terminal units but are advantageously internal units. Advantageously, the formula I fragments from 10 to 25, more preferably from 10 to 20 and most preferably from 11 to 16 mole percent of polymer (I). Advantageously, units of the formula II are up to 7.5, preferably from 0.3 to 7.5, and more preferably from 3.5 to 7.0 mole percent of the polymer (II).
In the copolymer having units of formula X, as defined above, units of the formula X is preferably from 5 to 40 mole percent of the copolymer, more preferably from 7.5 to 35 mole percent, most preferably from 7.5 to 25 mole percent. Such copolymer advantageously has a number average molecular weight as measured by gel permeation chromatography, of not more than 14,000, preferably from 2000 to 5500 and most preferably from 3000 to 4000.
The copolymer (I) preferably has a number average molecular weight as measured by gel permeation chromatography, of at most 14,000, advantageously at most 10,000, preferably from 1400 to 7000, more preferably from 2,000 to 5,500 and most preferably about 4000. For the polymer (II ) average molecular weight preferably does not exceed 20,000, preferably to 15,000, more preferably from 1,200 to 10,000 and most preferably from 3000 to 10000. The preferred number average molecular weight to some extent will depend on the number of carbon atoms in R 3 and R 4, and the more above this number, the higher the preferred molecular weight within the range specified above. The number average molecular weight of the polymer (II) preferably exceed at least 500, preferably 1000, number average molecular weight of the polymer (I).
Polymers in which R 1 and R 2 represents OOCR 4, are preferred and more preferably both, and R 1 and R 2 represents OOCR 4.
Polymers containing units I and fragments II, are preferably in a weight ratio of from 10: 1 to 1:10, preferably 10: 1 to 1: 3 and more preferably from 7: 1 to 1: 1.
The present invention relates to the use of two or more polymers (I) and / or two or more polymers (II) in the same additive composition. The present invention relates to a polymer and the use of (I) or (II), having two or more different types of fragments I and II. Fragments I in polymer (I) may be the same as or different from units I in polymer (II).
The oil-soluble copolymer of ethylene may comprise a copolymer of ethylene and at least one a-olefin having an average molecular weight of at least 30000. Preferably the a-olefin has at most 20 carbon atoms. Examples of such olefins are propylene, 1-butene, isobutene, n-octene-1, isooctene-1, n-decene-1 and n-dodecene-1. The copolymer may contain small amounts, e.g., up to 10 wt.%, Of other copolymerizable monomers, for example olefins other than the a-olefins and dienes with non-conjugated double bonds. The preferred copolymer is an ethylene-propylene copolymer. The present invention and include two or more different ethylene-a-olefin copolymers of this type.
The number average molecular weight of the ethylene-a-olefin copolymer is, as indicated above, at least 30,000, as measured by gel permeation chromatography (GPC) relative to polystyrene standards, advantageously at least 60,000 and preferably at least 80000. Functionally no upper limit, but at a molecular weight above about 150,000 movement difficulties arise as a result of increase in viscosity; the preferred molecular weight is between 60,000 and 80,000 to 120,000.
Advantageously, the molar ethylene content of the copolymer is from 50 to 85%. More preferably, the ethylene content - in the range from 57 to 80%, more preferably from 58 to 73%; even more preferably from 62 to 71% and most preferably from 65 to 70%.
Preferred ethylene-a-olefin copolymers are ethylene-propylene copolymers with a molar ethylene content of from 62 to 71% and a number average molecular weight ranging from 60,000 to 120,000; thus especially preferred copolymers are ethylene-propylene copolymers with an ethylene content of 62 to 71% and a molecular weight of from 80,000 to 100,000.
The copolymers may be prepared by any known method, for example using a Ziegler catalyst. The polymers should be substantially amorphous, since highly crystalline polymers are relatively insoluble in fuel oil at low temperatures.
The composition may include ethylene-a-olefin copolymer, advantageously with a number average molecular weight of at most 7500, advantageously from 1,000 to 6,000, and preferably 2,000 to 5,000, as measured by vapor phase osmometry. Suitable a - olefins are as given above, or styrene, with preference given to propylene. Advantageously the ethylene content is from 60 to 77 mole per cent although for ethylene-propylene copolymers can be employed with advantage in an amount of ethylene to 86 mole percent.
The copolymer should preferably be oil-soluble to the extent of at least 1000 ppm by weight. / Mn. by weight at a normal temperature. However, at least some of the copolymer may come out of solution near the cloud point of the oil and function to modify the wax crystals.
The composition advantageously contains the ethylene copolymer or copolymer blend in a total proportion of 0.0005 to 1, preferably from 0.001 to 0.5 and preferably from 0.01 to 0.15 wt.% Relative to the weight of fuel.
(B) comb polymers
Component (b) it is a comb polymer. Such polymers are discussed in "Comb-Like Polymers, Structure and Properties", NA Plate and VP Shibaev, J. Poly. Sci. Macromolecular Revs., Pp. 117-253 (1974).
Generally, comb polymers have one or more long chain branches such as hydrocarbyl branches having from 10 to 30 carbon atoms, hanging on the polymer backbone, said branch or branches being bonded directly or indirectly to the backbone. Examples of indirect bonding include bonding via interposed atoms or groups, this bonding can include covalent and / or ionic bonding such as in a salt.
Advantageously, the comb polymer is a homopolymer or copolymer having at least 25, preferably at least 40, more preferably at least 50 mole percent of the fragments with side chains containing at least 6, preferably at least 10, atoms, selected from for example carbon nitrogen and oxygen, in a linear chain.
As examples of preferred comb polymers include polymers of general formula 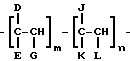
where D = R 11, COOR 11, OCOR 11, R 12 COOR 11 or OR 11;
E = H, CH 3, D or R 12;
G = H or D,
J = H, R 12, R 12 COOR 11, or an aryl or heterocyclic group,
K = H, COOR 12, OCOR 12, OR 12 or COOH,
L = H, R 12, COOR 12, OCOR 12, COOH, or aryl,
R 11i C 10 hydrocarbyl,
R 12i C 1 hydrocarbyl,
m and n are molar ratios, where m is in the range from 1.0 to 0.4, n - 0 to 0.6. R 11 advantageously represents a hydrocarbyl group with carbon number from 10 to 30; while R 12 advantageously represents a hydrocarbyl group with carbon number from 1 to 30.
If desired or necessary, the comb polymer may contain units derived from other monomers. The present invention includes one or more different comb polymers.
The molecular weight of the comb polymer is not critical. Preferably however it is in the range of from 1,000 to 100,000, preferably between 1,000 and 30,000 as measured by vapor phase osmometry.
These comb polymers may be copolymers of maleic anhydride or fumaric acid and another ethylenically unsaturated monomer, for example, a-olefin or an unsaturated ester, for example vinyl acetate. Preferably, but not necessarily, employ equimolar amounts of the comonomers, although acceptable molar ratios between 2: 1 and 1: 2. Examples of olefins that may be copolymerized. for example, maleic anhydride, include 1-decene, 1-dodecene, 1-tetradecene, 1-hexadecene and 1-octadecene.
The copolymer can be converted, e.g., esterified, by any suitable method, for example, by reaction with alcohols, primary or secondary amines or amino alcohols, and although preferred it is not essential that the maleic anhydride or fumaric acid be at least 50%. Examples of alcohols which may be used include n-decan-1-ol, n-dodecan-1-ol, n-tetradecan-1-ol, n-hexadecan-1-ol, and n-octadecan-1-ol. Alcohols may include up to one methyl branch per chain, for example, 1-methylpentadecan-1-ol, 2-methyltridecan-1-ol. The alcohol may be a mixture of the alcohol from normal alcohol to a methyl branched. Preferred to use pure alcohols rather than the commercially available alcohol mixtures but in the case of using mixtures of alcohols R 12 means the average number of carbon atoms in the alkyl group; if alcohols that contain a branch at the 1 or 2 positions are used R 12 refers to a straight segment of the alcohol chain.
These comb polymers may, in particular, polymers and copolymers itaconate or fumarate, such as those for example described in European patent applications 153176, 153177, 155807, 156577 and 225688 and International Patent Application WO 91/16407.
Particularly preferred fumarate comb polymers are copolymers of alkyl fumarates and vinyl acetate, in which the alkyl groups have from 12 to 20 carbon atoms, more especially polymers in which the alkyl groups have 12 carbon atoms or in which the alkyl groups are a mixture of C 12 / C 14 alkyl groups, obtained, for example, by solution copolymerizing an equimolar mixture of fumaric acid and vinyl acetate and reacting the resulting copolymer with the alcohol or mixture of alcohols, which are preferably straight chain alcohols. When using the mixture preferably to include normal C 12 and C 14 alcohols in the ratio 1: 1. Preferably, and use a mixture of C 12 -esters with the mixed C 12 / C 14 -esters. In such mixtures the weight ratio of C 12 to C 12 / C 14 is advantageously in the range from 1: 1 to 4: 1, preferably 2: 1 to 7: 2, and more preferably about 3: 1.
Other suitable comb polymers are the polymers and copolymers of a-olefins and esterified copolymers of styrene and maleic anhydride, and esterified copolymers of styrene and fumaric acid; a mixture of two or more comb polymers may be used in accordance with the present invention and, as indicated above, such use may be advantageous.
The composition advantageously contains the comb polymer in a proportion of 0.0005 to 1, preferably from 0.001 to 0.5, and most preferably from 0.01 to 0.15 wt.% Of the fuel mass.
(C) polar nitrogen compounds
Can be used, for example, one or more of the compounds (I) - (III):
(I) An amine salt and / or amide obtainable by treating at least one molar proportion of a hydrocarbyl amine with a molar proportion of a hydrocarbyl mono- or polycarboxylic acids, for example having 1 to 4 carboxylic acid groups or an acid anhydride.
There may be used an ester / amides containing from 30 to 300, preferably from 50 to 150 carbon atoms. These nitrogen compounds are described in U.S. Patent N 4211534. Suitable amines are usually long chain C 12 -C 40 primary, secondary, tertiary or quaternary amines or mixtures thereof but may be used with amines and shorter chain, provided that the resulting nitrogen compound oil soluble and accordingly normally contains from 30 to 300 carbon atoms. The nitrogen compound preferably contains at least one straight chain C 8 -C 40, preferably C 14 -C 24 alkyl segment.
Suitable amines include primary, secondary, tertiary or quaternary, but preferably are secondary. Tertiary and quaternary amines only form amine salts. Examples of amines include tetradecyl amine, cocoamine, and hydrogenated tallow amine fat. Examples of secondary amines include dioctadecyl amine and methyl.
and suitable mixtures of amines, such as those derived from natural substances. A preferred amine is a secondary hydrogenated tallow diamine, tallow having alkyl groups derived from hydrogenated tallow fat composed of approximately 4% C 14, 31% C 16% C 59 and 18 -radicals.
Examples of suitable carboxylic acids and their anhydrides for preparing the nitrogen compounds include cyclohexane 1,2 dicarboxylic acid, cyclohexene-1,2-dicarboxylic acid, cyclopentane-1,2-dicarboxylic acid and naphthalene dicarboxylic acid and 1,4-dicarboxylic acids including dialkyl spirobislactone. Generally, these acids have from 5 to 13 carbon atoms in the cyclic moiety. Preferred acids are benzene dicarboxylic acids such as phthalic acid, isophthalic acid and terephthalic acid. Phthalic acid or its anhydride is particularly preferred.
Preferred compounds are the amide-amine salt of phthalic anhydride with two molar proportions of hydrogenated tallow amine fat, the diamide product obtainable by dehydrating this salt, and the amide-amine salt of ortho-sulphobenzoic anhydride and hydrogenated tallow amine fat.
Other examples are long chain alkyl or alkylene substituted dicarboxylic acid derivatives such as amine salts or monoamides of substituted succinic acids, examples of which are described for example in U.S. Patent 4,147,520 Suitable amines may be those described above. Other examples are condensates such, for example as described in EP-A-327 423, EP-A-413279 and EP-A-398101.
(II) A compound consisting of or including a ring system, the compound carrying on the ring system at least two but preferably only two substituents of the general formula
-A-NR 21 R 22
where A is an aliphatic hydrocarbyl group that is not necessarily interrupted by one or more hetero atoms and that is straight chain or branched, and R 21 and R 22 are identical or different and each is independently a hydrocarbyl group containing 9 to 40 carbon atoms, not necessarily interrupted by one or more hetero atoms, the substituents being identical or different and the compound optionally being in the form of its salt, e.g., hydrochloride or acetate.
Preferably, A has from 1 to 20 carbon atoms and is preferably a methylene or polymethylene group.
The cyclic ring system may be a homocyclic, heterocyclic, monocyclic, polycyclic or fused polycyclic assembly or a system where two or more such cyclic assemblies are joined to one another and in which the cyclic assemblies may be the same or different. Where there are two or more such cyclic assemblies, the substituents may be specified in the same or different assemblies, preferably in the same ensemble. Preferably, the or each cyclic assembly is aromatic cyclic ensemble, more preferably a benzene ring. Most preferably the ring system is a single benzene ring, the substituents are preferably in the ortho or meta positions, which benzene ring may be optionally further substituted.
The ring atoms in the cyclic assembly or assemblies are preferably carbon atoms but may for example include one or more ring N, S or O.
Examples of such polycyclic assemblies include:
(A) condensed benzene structures such as naphthalene, anthracene, phenanthrene, and pyrene;
(B) condensed ring structures where none or one of them, or all of the rings are benzene, for example, azulene, indene, hydroindene, fluorene, and difenilenoksid;
(C) rings joined "end-on" such as diphenyl;
(D) heterocyclic compounds such as quinoline, indole, 2,3-dihydro-indole, benzofuran, coumarin, isocoumarin, benzothiophene, carbazole and thiodiphenylamine;
(E) non-aromatic or partially saturated ring systems such as decalin (decahydronaphthalene), alpha-pinene, cardinene and bornylene; and
(E) multi-ring structures, for example, norbornene, bicycloheptane (norbornane), bicyclooctane and bicyclooctene.
Each hydrocarbyl group R 21 and R 22 may, for example, be an alkyl or alkylene group or a mono- or polyalkoxyalkyl group. Preferably, each hydrocarbyl group is a linear alkylene group. The number of carbon atoms in each hydrocarbyl group is preferably from 16 to 40, more preferably from 16 to 24.
The compounds may conveniently be made by reducing the corresponding amide which may in turn be prepared by reacting the appropriate secondary amine and acid chloride.
(III) The condensation products of long chain primary or secondary amine with a polymer containing carboxylic acid.
Specific examples include polymers such as described in GB-A-2121807, FR-A-1535723 and DE-A-3941561; and telomeric acids and esters and alkanolamines as described in US Patent No. 4639256; and the reaction product of an amine containing a branched carboxylic acid ester, an epoxide and a monocarboxylic acid polyester as disclosed in U.S. Patent 4,631,071.
Compounds containing at least one comb polymer and / or at least one polar nitrogen compound in addition to ethylene and an unsaturated ester copolymer have much improved resistance to wax deposition and are preferred.
(G) The compounds according to the description
By "substantially linear" means that the alkyl group is preferably straight chain, but nevertheless can be used and mostly straight-chain alkyl groups having a small degree of branching such as in the form of a single methyl group.
Preferably the compound has at least two of said alkyl groups when the linear chain may include the carbon atoms of more than one of said alkyl groups. When the compound has at least three of said alkyl groups may be more than one of such linear chains, which chains may overlap. The linear chain or chains may provide part of a linking group between any two such alkyl groups in the compound.
The oxygen atom or atoms are preferably directly implanted between the carbon atoms in the chain and may, for example, be in the form of mono- or polyoxyalkylene group. Examples of said oxyalkylene group preferably having 2 to 4 carbon atoms include oxyethylene and oxypropylene.
As indicated the chain or chains include carbon and oxygen atoms. They may comprise other heteroatoms, such as nitrogen.
The compound may be an ester where the alkyl groups attached to the remainder of the compound, such as -O-CO-n-alkyl or -CO-O-n-alkyl group, in the first case the alkyl groups are derived from an acid and a compound residue derived a polyhydric alcohol and in the latter the alkyl groups are derived from an alcohol and a compound residue derived from a polycarboxylic acid. and the compound may be an ether where the alkyl groups attached to the remainder of the compound, such as n-O-alkyl group. The compound may be both an ester and an ether or it may contain different ester groups.
Examples include polyoxyalkylene esters, ethers, ester / ethers and mixtures thereof, particularly those containing at least one, preferably at least two C 10 -C 30 linear alkyl groups and a polyoxyalkylene glycol group of molecular weight up to 5000, preferably from 200 to 5,000, the alkylene group in said polyoxyalkylene glycol containing from 1 to 4 carbon atoms, as described in EP-a-61895 and U.S. Patent 4,491,455.
The preferred esters, ethers or ester / ethers which may be used may be structurally displayed formula
R 23 OBOR 24,
where R 23 and R 24 are identical or different and may be
(A) n-alkyl,
(B) n-alkyl-CO-,
(c) n-alkyl-OCO- (CH 2) n-,
(d) n-alkyl-OCO- (CH 2) n CO-,
n is, for example, from 1 to 34, the alkyl group being linear and containing from 10 to 30 carbon atoms and B represents the polyalkylene segment of the glycol in which the alkylene group has from 1 to 4 carbon atoms, such as polyoxymethylene, polyoxyethylene or polyoxytrimethylene parts which is substantially linear; some degree of branching with side chains of the lower alkyl can be tolerated (such as in polyoxypropylene glycol), but it is preferred the glycol should be substantially linear. B and may contain nitrogen.
Suitable glycols generally are substantially linear polyethylene glycols (PEG) and polypropylene glycols (PPG) having a molecular weight from about 100 to about 5000, preferably from about 200 to 2000. The esters are preferred, and for reacting with the glycols use fatty acids having 10 to 30 carbon atoms to form the ester additives, it being preferred for use are c 18 -C 24 fatty acid, especially behenic acid. Esters may be prepared by esterifying polyethoxylated fatty acids or polyethoxylated alcohols.
Polyoxyalkylene diesters, diethers, ether / esters and mixtures thereof are suitable as additives, diesters being preferred when the base oil component is a fraction having a narrow boiling range, which may be present minor amounts of monoethers and monoesters (which are often formed in the manufacturing process). For active performance is important that a major amount of the dialkyl compound. In particular, stearic or behenic diesters of polyethylene glycol, polypropylene glycol or mixtures / polypropylene are preferred.
Examples of other compounds in this general category are those described in Japanese Patent Publication 2-51477 and 3-34790 and EP-A-117108 and EP-A-326,356, and cyclic esterified ethoxylates such as described EP-A- 356,256.
The composition may contain other additives for improving low temperature and / or other properties, many of which are used in the art or known from the literature.
The scope of the invention and provides an additive concentrate comprising the additive in admixture with a biofuel or with a liquid mixture of a biofuel and petroleum-based fuels. The invention further provides the use of additives for improving low temperature properties of the mixtures biofuel / petroleum-based fuel.
The following examples in which all parts and percentages are by weight, number average molecular weight measured by vapor phase osmometry, and internal methyl groups in polymers by proton NMR (i.e., excluding terminal methyl groups and those that are on the acetate groups), illustrate the invention.
Hydrocarbon fuels used in the Examples had the characteristics indicated at the end of the description.
Rapeseed oil methyl ester obtained by extraction from seeds using a screw press, refining, and transesterifying with methanol.
example 1
In this example, the biofuel used was an RME with a cloud point temperature of -4 o C and CFPP -11 o C, and the petroleum fuel was Fuel 2.
Ethylene-unsaturated ester copolymer is a mixture of two ethylene-vinyl acetate copolymers.
EVA 1.36 wt.% Vinyl acetate, ![]() about 2400, CH 3/100 CH 2 Apr. and
about 2400, CH 3/100 CH 2 Apr. and
EVA 2, 14 wt.% Vinyl acetate, ![]() approximate 3500, CH 3/100 CH February 7.
approximate 3500, CH 3/100 CH February 7.
The weight ratio of EVA 1: EVA 21 is 6: 1.
Substance that prevents paraffin deposition (WASA) is WASA 1, which is a mixture of equal parts by weight of C 1 / C 14 -alkilfumarata / vinyl acetate comb polymer and the amide-amine salt of phthalic anhydride with two molar proportions of hydrogenated tallow.
320 ppm. / Million polymer blend of EVA 1 and EVA 2 were mixed with pure RME, pure Fuel 2, and mixtures of RME and Fuel 2, and CFPP value is compared with the values for the untreated fuels. The results are shown in Table. 1.

From these results it can be seen that while the EVA blend was only marginally effective in a RME, and showed its usual effect on the CFPP of fuel oil, CFPP values of the treated RME / Fuel 2 mixtures were substantially reduced.
Further samples as described above, untreated and treated with, in addition to various concentrations of the EVA blend, various concentrations of WASA 1, and stored at -15 o C for 3 days. They were then assayed for wax formation, its appearance and degree of settling if present, and the transparency of the liquid. The results are shown in Table. 2, together with the values of CFPP materials.
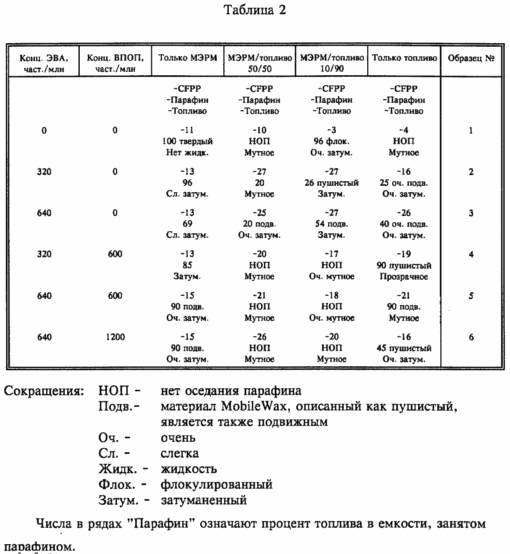
In all tables, the concentration of additives is given in terms of active ingredient actually used.
The results show that the combination of EVA and WASA in fuel mixtures effectively reduces CFPP and prevents wax settlement.
example 2
In this example, the fuel is a petroleum-based fuel; the same RME was used as in Example 1.
As well as the blend of EVA 1 and EVA 2 used in Example 1, an ethylene-vinyl acetate copolymer containing 29 wt.% Vinyl acetate, ![]() about 2400, CH 3/100 CH 2 4, this is denominated EVA 3. WASA 2 is a blend of equal parts by weight of C 12 - alkyl fumarate / vinyl acetate comb polymer and the same amide-amine salt as in WASA 1. WASA 3 is a blend of one part by weight of each component, C 16 and C alkyl polyitaconate alkyl polyitaconate 18, and two parts by weight of the same amide-amine salt as in WASA 1.
about 2400, CH 3/100 CH 2 4, this is denominated EVA 3. WASA 2 is a blend of equal parts by weight of C 12 - alkyl fumarate / vinyl acetate comb polymer and the same amide-amine salt as in WASA 1. WASA 3 is a blend of one part by weight of each component, C 16 and C alkyl polyitaconate alkyl polyitaconate 18, and two parts by weight of the same amide-amine salt as in WASA 1.
Samples described in Table. 3 below were tested for CFPP and for appearance after storage for 4 days at -15 o C.
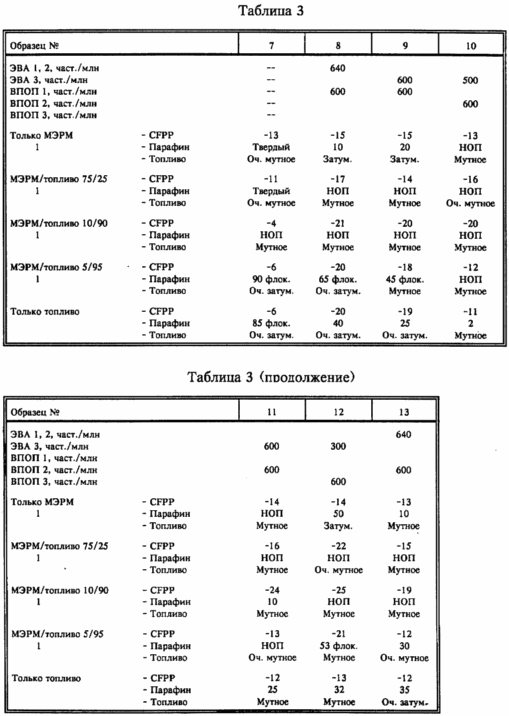
From the results table. 3 shows that in many cases the improvement in CFPP and reduction in wax settlement are better for the mixtures than for the individual fuels.
example 3
In this example, Fuel 2 was used together with the same RME as used in Example 1. The results of Examples 1 and 2 are confirmed. The results are shown in Table. 4.
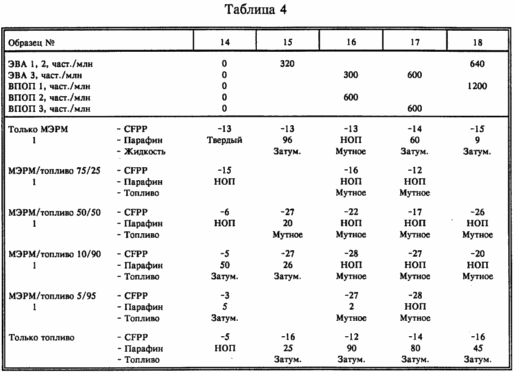
example 4
In this example, the biofuel used as in Example 1, and the petroleum fuel was Fuel 2.
600 част./млн фумарат-винилацетатного гребнеобразного сополимера смешивают с чистым МЭРМ, с чистым топливо 2 и смесью МЭРМ и топлива 2 и значения CFPP сравнивают с таковыми для необработанных топлив.
Сополимер является смешанным C 12 /C 14 алкилфумаратом, полученным путем реакции смеси 1:1 по весу нормальных C 12 и C 14 спиртов с фумаровой кислотой, и винил-ацетатным сополимером, полученным путем полимеризации в растворе. The results presented in Table. 5, показывают, что чистый гребнеобразный полимер является неожиданно эффективным при понижении CFPP смеси нефти и биотоплив.

и были приготовлены концентраты А и Б, содержащие:
50 мас.% присадок, понижающих температуру текучести топлив на холоде.
Были взяты следующие присадки:
(I) этилен-винилацетатный сополимер.
(II) C 22 сложный эфир полиэтиленгликоля /PEG 200/400/600 (фирмы Croda Chemical).
(III) амид-аминная соль, полученная из фталевого ангидрида и двух молярных частей гидрированного таллового амина.
(IV) сополимер фумарового сложного эфира и винилацетата.
Указанные присадки (I-IV) разбавляли растворителем в следующей концентрации.
Концентрат A:
50 мас.% метилового эфира рапсового масла в качестве биотоплива.
Концентрат Б:
50 мас.% смеси 5 мас.% метилового эфира рапсового масла в качестве биотоплива с дизельным топливом.
CLAIM
1. Состав жидкого топлива на основе смеси биотоплива с нефтяным топливом, отличающийся тем, что он дополнительно содержит одну или более присадок, модифицирующих кристаллы парафина и/или понижающих температуру текучести топлив, выбранных из групп: маслорастворимый сополимер этилена, гребнеобразный полимер; полярное соединение азота; соединение, в котором по крайней мере одна в основном линейная алкильная группа, имеющая 10 - 30 атомов углерода, присоединена к неполимерному органическому остатку с образованием по крайней мере одной линейной цепочке атомов, включающей атомы углерода указанных алкильных групп и один или более неконцевых атомов кислорода.
2. Состав по п.1, отличающийся тем, что в качестве биотоплива содержит растительное масло или переэтерифицированное растительное масло.
3. Состав по п.2, отличающийся тем, что в качестве биотоплива содержит рапсовое масло или его производное.
4. Состав по п.2, отличающийся тем, что в качестве биотоплива содержит метиловый эфир рапсового масла.
5. Состав по пп.1 - 4, отличающийся тем, что в качестве нефтяного топлива содержит среднюю фракцию перегонки нефти.
6. Состав по пп.1 - 5, отличающийся тем, что в качестве нефтяного топлива содержит дизельное топливо.
7. Состав по пп.1 - 6, отличающийся тем, что содержит 5 - 75 мас.% биотоплива от смеси топлив.
8. Состав по пп.1 - 7, отличающийся тем, что в качестве маслорастворимого сополимера этилена содержит сополимер этилена со сложным эфиром с ненасыщенными этиленовыми связями.
9. Состав по пп.1 - 7, отличающийся тем, что в качестве маслорастворимого сополимера этилена содержит сополимер этилена и сложного эфира ненасыщенного спирта и насыщенной карбоновой кислоты.
10. Состав по пп.1 - 9, отличающийся тем, что в качестве маслорастворимого этиленового сополимера содержит сополимер, имеющий в дополнение к фрагментам, являющимся производными этилена, фрагменты формулы
-CH 2 - CRR 30 -,
где R представляет собой H или CH 3 ;
R 30 представляет собой группу формулы COOR 3 или OOCR 4 , где R 3 и R 4 независимо представляют собой гидрокарбильную группу.
11. The composition according to claims 1 - 10, characterized in that it comprises two oil-soluble ethylenically unsaturated copolymer, of which one has a higher molecular weight and a lower ester content than the other.
12. The composition of claim. 11, characterized in that it comprises (I) in the oil-soluble ethylene copolymer having, in addition to units derived from ethylene, from 7.5 to 35 mole percent of units of the formula
-CH 2 - CRR 1 -
and (II) in the oil-soluble ethylene copolymer having, in addition to units derived from ethylene, up to 10 mol.% units of formula
-CH 2 - CRR 2 -,
wherein each R independently represents H or CH 3;
each R 1 and R 2 independently represents a group of the formula COOR 3 or OOCR 4, wherein R 3 and R 4 independently represent a hydrocarbyl group,
wherein the proportion of units I in polymer (I) at least 2 mol.% higher than the proportion of units II in polymer (II).
13. The composition according to claims 1 - 12, characterized in that it contains the additive in an amount of 0.0005 - 1.0 wt.%.
14. The composition according to claims 1 - 13, characterized in that the polymer comprises a comb polymer of the general formula 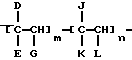
where D - R, COOR 11, OCOR, R 12 COOR 11 or OR 11;
E - H, CH 3, D or R 12;
G - H or D;
J - H, R 12, R 12 COOR 11, or an aryl or heterocyclic group;
K - H, COOR 2, OCOR 12, OR 12 or COOH;
L - H, R 2, COOR 2, OCOR 12, COOH, or aryl;
R 11 - C 10 hydrocarbyl;
R 12 - C 1 hydrocarbyl;
m and n represents a molar ratio, m is in the range from 1.0 to 0.4, n ranges from 0 to 0.6.
15. The composition of claim. 14, characterized in that the comb polymer comprises a copolymer of vinyl acetate and a fumarate ester.
16. The composition according to claim 15, characterized in that it comprises a copolymer of vinyl acetate and alkyl fumarate carbon atoms in the alkyl group of 12 - 20.
17. The composition according to claim 16, characterized in that it comprises a copolymer in which the ester groups are derived from C 12 alcohol or a mixture of C 12 -C 14 alcohols.
18. The additive concentrate of modifying the wax crystals and / or lowering the temperature of fuel flow through the solvent, characterized in that as additives contains additives according to claims 1 - 17 and comprises a solvent or a mixture biofuels with petroleum fuels.
print version
Publication date 07.04.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.