| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2241656
![]()
METHOD OF STORAGE AND PRODUCTION OF HYDROGEN BY MAGNESIUM HYDROLYSIS FOR AUTONOMOUS ENERGY INSTALLATIONS WITH ELECTROCHEMICAL GENERATORS
The name of the inventor: Avakov V.B.
(RU); Zinin V.I. (RU); Ivanitsky B.A. (RU); Kulakov G.V. (RU); Landgraf I.K. (RU); Khrestinin M.M. (RU)
The name of the patent holder: Federal State Unitary Enterprise "Central Research Institute of Shipborne Electrical Engineering and Technology" (RU)
Address for correspondence: 196128, St. Petersburg, ul. Blagodatnaya, 6, Federal State Unitary Enterprise "Central Research Institute of Ship Electrical Engineering and Technology"
Date of commencement of the patent: 2003.01.21
The invention relates to a method for storing and producing hydrogen in autonomous power plants with electrochemical generators with a cycle of operation from a few hours to several thousand hours. The process involves the production of hydrogen by hydrolysis of magnesium when the water is supplied as a saturated or superheated steam. Magnesium is used in the form of sheet, wire, granules of regular or irregular shape, with the condition that one of the linear dimensions of the mold used does not exceed 1-2 mm. For the reaction, water generated in the electrochemical generator is used, and the heat released during the reaction itself is used. In power plants with low autonomy, up to several hours, hydrolysis is carried out at a temperature of 400-450 ° C, while using a container method of storage and replacement of the entire spent container. In power plants with greater autonomy, the process is carried out at 120-150 ° C, non-replaceable containers are used, and the reaction products are removed by treating magnesium oxide with an acid, for example hydrochloric acid, to obtain a magnesium salt, its subsequent dissolution and extrusion from the container. The flow rate of the hydrogen produced is controlled by adjusting the amount of water supplied as a vapor. In order to reduce the energy required to prepare for the hydrolysis reaction, the magnesium container (store) is partitioned and one or more of the sections is used as a "primer". The technical result consists in a significant increase in the mass percentage of the hydrogen produced, provided that there is no replenishment from the outside of the starting products and the release of reaction products during the autonomy period.
DESCRIPTION OF THE INVENTION
The invention relates to the field of autonomous power plants, mainly with electrochemical generators.
A distinctive feature of autonomous power plants (EI) is the periodicity of their functioning during a relatively short time, the duration of which is determined by the reserves of reagents (fuel and oxidizer).
Such facilities include installations for submarines, underwater vehicles, ships, rail and road transport, household energy sources of periodic action, as well as periodically operating stationary power plants used in critical facilities that do not allow power interruption.
The way of storing and obtaining hydrogen in autonomous EC should ensure its long-term and safe storage, and its safe production at the minimum cost, mass and volume of the hydrogen storage and production system, the simplicity of the operation of the EA and the utilization of the reaction products.
The following methods of storage and production of hydrogen for autonomous power plants are known (see Lidorenko NS, Muchnik GF Electrochemical generators, M., 1982, Korovin NV Electrochemical energy .- M .: Energoatomizdat, 1991) :
- storage in a gaseous state, in which hydrogen is stored in vessels under high pressure (up to 50 MPa) and after throttling is fed to an electrochemical generator (ECG);
- storage in a liquid state (cryogenic), at which hydrogen is gasified before being supplied to ECG;
- storage in the composition of intermetallic compounds, in which it is pre-sorbed, and before being fed to the ECG, it is desorbed with heat absorption;
- storage of hydrogen in a chemically bound state in the composition of hydrogen-containing compounds, when hydrogen is produced by an appropriate chemical process.
The last method of storage and production of hydrogen includes:
- storage of hydrogen in the composition of ammonia and its production by dissociation;
- storage of hydrogen in the composition of methanol and other liquid hydrocarbons and its production by steam or steam-oxygen conversion thereof;
- storage of hydrogen in the composition of metal hydrides and its production by thermal decomposition;
- storage of hydrogen in the composition of hydrides of metals and water and its production by hydrolysis of metal hydrides.
None of the above methods of storage and production of hydrogen does not meet all the requirements for storage and production of hydrogen for autonomous EC.
The method of storage in intermetallic compounds is the safest and most convenient to use, but it is expensive to manufacture and predetermines a large mass of EC since the cost of 1 kg of intermetallic compound is $ 15-35, and the mass hydrogen sorbents of widely used sorbents is only 1.5-2.0% .
The lowest mass and volume of the installation can be obtained with a sufficient level of safety using methanol or hydrocarbon fuel conversion, but gaseous reaction products are inevitable, which in some cases are unacceptable (in submarines and other similar objects), since Can lead to the loss of stealth.
There are also known methods for producing hydrogen by the interaction of water with aluminum and magnesium.
But the hydrolysis of aluminum by water takes place only in the presence of alkali according to one of the reactions listed below
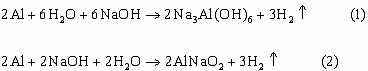
The mass yield of hydrogen according to the reaction (1) is about 1.5%, and the product of the reaction is insoluble in water, therefore further consideration of reaction (1) is of no interest.
The mass yield of reaction (2) theoretically reaches 3.5%, the product of the reaction is limitedly soluble in water, therefore, in order to be able to remove the reaction products after the end of the operation cycle, they are usually stored as a solution, which requires additional water, which can reduce the mass Hydrogen yield up to 1.5-2%.
This circumstance, taking into account the irreversibility of the process, makes reaction (2) uninteresting.
The hydrolysis of magnesium with water can proceed according to the reaction
![]()
The mass yield of hydrogen is 3.3%, and the product of the reaction is insoluble in water.
The present invention is directed to significantly increase the mass percentage of the hydrogen produced relative to the total weight of the reaction starting materials, provided that the replenishment from the outside of the starting products, for example water, and the release of the reaction products in the autonomy period, is absent, which is very important for providing Concealment of an object equipped with EC with ECG.
The experiments carried out showed that under certain conditions the process can be realized by a reaction different from (3), namely
![]()
As a result of the reaction of magnesium with water, pure magnesium oxide and hydrogen are obtained. At the same time, the mass yield of hydrogen is more than 4.7%, and if we take into account that the amount of water required for the reaction is small and equal to that generated in the ECG during the operation of the EA, then using it, the mass yield of hydrogen reaches 8.23%.
An obligatory condition for the realization of (4) is the supply of water in the vapor phase, in the form of saturated or superheated steam in an amount close to the stoichiometry. In this case, the amount of hydrogen liberated is controlled by the amount of water supplied.
Magnesium can be used in any form: sheet, wire, granules of regular and irregular shape, and the like. It is important to ensure the maximum reaction surface and its completeness.
In both cases, this assumes that one of the linear dimensions of the mold used is sufficiently small and does not exceed 1-2 mm.
The level of temperatures at which it is expedient to carry out the reaction, but also the method of storage of the initial products and the way of substituting the reaction products for the initial ones depend on the type of power plant and its purpose.
In power plants with a relatively small autonomy, which does not exceed several hours, for example in road transport ECs, it is advisable to use a container method of storage and replacement, and the reaction should be carried out at a temperature of 400-450 ° C. In this process, magnesium in the form of sheet, wire, granules of regular or irregular shape is placed in special containers in which the reaction of oxidation of magnesium with steam with intensive release of hydrogen is carried out. After all the magnesium in the container reacts with water vapor and turns into magnesium oxide, the container from the plant is extracted and sent to a specialized enterprise, and a new container with magnesium is installed in the place of the spent container.
In power plants with large autonomy, which amounts to tens, hundreds and thousands of hours (for example, in submarine power plants) for magnesium storage, it is advisable to use non-replaceable containers (storages), and oxidation of Mg should be conducted at a temperature of 120-150 ° C. In this case, the removal of reaction products (magnesium oxide) from a stationary storage can be carried out under basic conditions according to the following scheme: preliminary treatment of magnesium oxide with acid to obtain a water-soluble magnesium salt; Dissolution of this salt; Extruding the solution from the container; Washing the container with water.
For this purpose, for example, the reaction
![]()
After purification from the reaction products, the container is filled with a fresh portion of Mg, which is expediently used in the form of granules or in any other form, which ensures the flowability of the material and the absence of fine dust that can lead to spontaneous combustion.
In order to ensure the initiation of reaction (4), it is necessary to obtain steam and to heat magnesium to a predetermined temperature. For this purpose, for example, any electrical device (heating pad, electric discharge, etc.), powered either from a basic source or from a source on board, can be used. Since the reaction is highly exothermic, it is important to create it and then maintain a predetermined temperature regime by removing excess heat.
In order to reduce the energy required to prepare for the magnesium hydrolysis reaction (steam production and Mg preheating to the desired temperature), it is advisable to partition the magnesium storage and have one or more "ignition" sections. In this case, only the "ignition" section is initially heated, and then the rest is heated by the heat released in it as a result of the reaction.
CLAIM
1. A method of storing and producing hydrogen in autonomous power plants with electrochemical generators with a cycle of operation from one to thousands of hours, mainly for power plants of submarines, underwater vehicles, motor vehicles and periodically operating stationary installations used in critical facilities that do not allow power interruption , Providing for the production of hydrogen by magnesium hydrolysis at an elevated temperature, characterized in that the hydrolysis of magnesium is carried out by feeding water in the form of saturated or superheated steam in an amount close to the stoichiometric according to reaction ![]() , Than provide the maximum mass yield of hydrogen.
, Than provide the maximum mass yield of hydrogen.
2. The method of claim 1, wherein the hydrolysis of magnesium uses water generated in the electrochemical generator.
3. The method of claim 1, wherein the heat generated by the hydrolysis of magnesium is used to generate water vapor and heat the magnesium to a predetermined temperature.
4. The method of claim 1, wherein adjusting the amount of hydrogen produced is provided by adjusting the amount of water supplied to the reaction.
5. The method of claim 1, wherein in order to reduce the energy required to prepare for the reaction, the magnesium storage is partitioned and one or more of the sections is used as a "primer".
6. The method according to claim 1, characterized in that for power plants with a relatively small autonomy not exceeding several hours, mainly automobile ones, the hydrolysis process is carried out at a temperature of 400-450 ° C., while magnesium in the form of a sheet, rolled or pellet is correct or Irregular shape, in which one of the linear dimensions of the mold used does not exceed 1-2 mm, are placed in special easily removable containers which, after completion of the reaction, are extracted from the unit and sent for processing of magnesium oxide to a specialized enterprise, and in its place a new container with magnesium .
7. The method according to claim 1, characterized in that for power plants with a relatively large autonomy of tens, hundreds and thousands of hours, primarily for power plants for submarines, non-replaceable containers (storages) are used to store magnesium, and the process of hydrolysis is carried out at a temperature of 120-150 ° C, while the removal of reaction products (magnesium oxide) is carried out under basic conditions after preliminary treatment of magnesium oxide with an acid solution, for example, hydrochloric acid ![]() , To obtain a water soluble magnesium salt and dissolving this salt in water, a method of expelling it from the container, followed by washing it and loading a new portion of magnesium in the form of granules or in any other form in which one of the linear dimensions of the mold used does not exceed 1- 2 mm, providing the flowability of the material.
, To obtain a water soluble magnesium salt and dissolving this salt in water, a method of expelling it from the container, followed by washing it and loading a new portion of magnesium in the form of granules or in any other form in which one of the linear dimensions of the mold used does not exceed 1- 2 mm, providing the flowability of the material.
print version
Date of publication 04.01.2007gg




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.