| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2191742
![]()
METHOD OF OBTAINING HYDROGEN
The name of the inventor: Adamovich BA; Derbichev Ahmet Giri Bamat Gireyevich; Dudov VI; Kim OD; Kobyakov DP; Trubitsyn A.P.
The name of the patent holder: Adamovich Boris Andreevich; Derbichev Ahmet Giri Bamat Gireyevich; Vladimir Dudov; Kim Oleg Davidovich; Kobyakov Dmitry Petrovich; Trubitsyn Alexander Pavlovich
Address for correspondence: 123557, Moscow, Bolshoy Tishinsky per., 8, p. 2, "Resursprominvest", VI Dudov
Date of commencement of the patent: 2000.08.31
The invention relates to the chemical industry, in particular to methods for producing particularly pure hydrogen gas. Hydrogen is produced from water vapor by converting it into a medium heated in a high-voltage discharge of technical iron, then subjected to a two-stage drainage and collection into intermetallic compressors, leading hydrogen to desorption to a high purity of 99.99% by volume.
DESCRIPTION OF THE INVENTION
The invention relates to the chemical industry, in particular to methods for producing particularly pure hydrogen gas.
Various methods for producing hydrogen gas are known:
(Technical Dictionary, Moscow: GONTI NKTP of the USSR, 1939, p.219):
- from water by electrolysis;
- from water by the conversion of water vapor in a medium of incandescent iron to iron oxides and hydrogen gas;
- from water gas (CO 2 + H 2 ) using various catalysts, including platinum and palladium;
- from coke oven gas by deep freezing of impurities.
A method is known for producing hydrogen by water-vapor conversion of carbon monoxide in the presence of a copper-zinc manganese catalyst (Russian patent 2,050,975, December 30, 1992).
However, the known methods do not provide high purity of hydrogen (99.99%).
The closest analogue is the method for producing hydrogen by conversion of steam in a hot iron to iron and hydrogen oxides (SU 1125186, published on November 23, 1984) in a reactor.
This method is characterized by high cost and does not provide high purity of hydrogen.
The invention is based on the chemical reaction of hydrogen production known for a long time (Petisson Muir, Chemistry of Fire, Moscow: Sabashnikov Publishing, 1899)
3Fe + 4H2O = Fe3O4 + 4H2.
This reaction occurs when high-purity iron is blasted by steam.
The object of the invention is to produce highly pure hydrogen (99.99%) using as a raw material for carrying out said reaction of technical iron according to OST-y 7123 containing 0.05-0.15% by weight of carbon and a number of other impurities, and applying a high-voltage Controlled discharge to maintain a high temperature of up to 1000 ° C in the reactor, this is the technical result of the invention.
The objective is achieved by a method for producing hydrogen by conversion of steam in a hot iron reactor to iron oxides and hydrogen gas in a reactor. A reactor is used consisting of a cooling jacket and a high-voltage spark gap with two electrodes, one of which is made of technical iron, distilled water is boiled in the tank to form saturated steam, it is fed to the cooling jacket of the reactor to form superheated steam, an alternating current 3.6 kW, simultaneously injected superheated steam through the nozzle into the discharge gap, and the formed iron oxides are shaken to vibration with a vibration into the collection tank, wet hydrogen is discharged from the reactor to a condenser cooled by water from the water supply system, the condensate is discarded, then the previously dried hydrogen is subjected Final drying in the regenerated silica gel cartridges, then hydrogen is passed through the microporous filter to consumers in intermetallic compressors, which, when desorbing hydrogen, ensure its purity to 99.99% by volume.
The figure shows a plant for producing hydrogen.
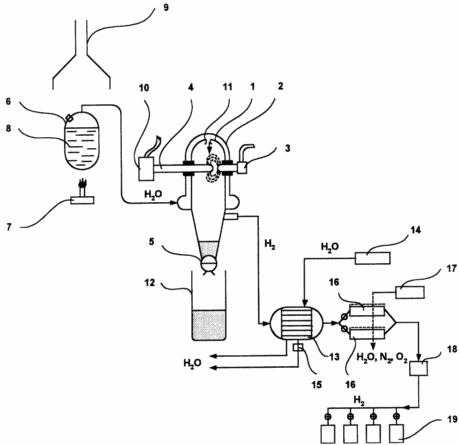 |
Take reactor 1, consisting of a cooling jacket 2, a high-voltage spark gap with two electrodes: 3 - a permanently fixed tungsten and 4 - an electrode made of engineering iron, which is inserted into the reactor, and a gate 5 to remove the higher iron oxides formed. In the tank 6, with the gas burner 7, distilled water 8 is boiled to form saturated steam and is fed into the cooling jacket 2 to form superheated steam and the burnt gases are discharged into the chimney 9. An alternating current of 3.6 is applied to the dischargers 3, 4 KW and by means of a regulator 10 electrode 4 is introduced until a discharge is formed, subjecting it to vibrational action to discharge the formed oxides. The saturated water vapor is overheated in the cooling jacket and through the nozzle 11 is directed to the discharge gap. The resulting iron oxides through the gate 5 are removed to the collection vessel 12. The wet hydrogen is discharged from the reactor to the condenser 13, which is cooled by water from the water supply system 14, and the condensate formed through the valve 15 is discharged. After the first drying step, hydrogen is introduced into the regenerated silica gel cartridges 16, the regeneration being carried out by heating the cartridges and blowing them dry with the air heater 17. Then, the dried hydrogen through the microporous filter 18 is dispensed to consumers in hydrogen intermetallic compressors 19 which, upon desorption of hydrogen Ensure its purity to 99.99% vol. |
Example (calculated)
In order to provide hydrogen productivity equal to 100 kg per year (350 g / h) under two-shift operation, the iron electrode is heated up to 800-1000 o C at a voltage of 3.6 kV, current strength up to 3A and power consumption up to 10 kW .
The consumed power is consumed to provide the heat of formation of iron oxides equal to 200 kcal / g-mole. Thus, for the production of 100 kg of hydrogen, 5 thousand kWh of electricity are consumed, taking into account the thermal losses, with a total cost of no more than 5,000 rubles, depending on the region.
Taking into account the cost of one gram of extremely pure hydrogen equal to $ 50 on the Merk catalog, they form an annual sales revenue of $ 5 million, with a favorable market situation facing a deficit in especially pure hydrogen used in thermonuclear research and in the work of numerous gas analytical laboratories .
CLAIM
A process for the production of hydrogen by conversion of steam in a hot iron to iron oxides and hydrogen gas in a steam reactor, characterized in that a reactor is used consisting of a cooling jacket and a high-voltage spark gap with two electrodes, one made of engineering iron, boiling distilled Water, forming saturated steam, it is fed into the cooling jacket of the reactor to form superheated steam, an alternating current of 3.6 kV is applied to the high-voltage spark gap, superheated steam is injected through the nozzle into the discharge gap, and the formed iron oxides are dumped into the collecting tank ; Moist hydrogen is discharged from the reactor into a condenser cooled by water from the water supply system, the condensate is discarded, then the previously dried hydrogen is finally dried in the regenerated silica gel cartridges, then hydrogen is passed through the microporous filter to consumers in intermetallic compressors that, when desorbed, ensure its purity to 99 , 99% v / v.
print version
Date of publication 26.02.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.