| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2050975
![]()
METHOD FOR PRODUCING HYDROGEN AND METHOD FOR PRODUCING CATALYST
To produce hydrogen
Name of the inventor: Kozlov I.L .; Pavelko VZ .; Firsov OP .; Kuznetsov AS
The name of the patentee: Research and Production Association "EMEKAT"
Address for correspondence:
Starting date of the patent: 1992.12.30
Usage: to processes for producing hydrogen by catalytic conversion of carbon monoxide, but also in compositions and methods for preparing the catalysts of this process. The inventive method comprises the production of hydrogen by steam reforming of carbon monoxide in the presence of copper oxide-zinc-manganese catalyst comprising, by weight. 30 65 copper-oxide; 18 31 zinc oxide; 0.1 2.7 MnO; 0.08 - 0.8 carbon and a carrier based on alumina rest. The process is carried out at 190 400 ° C and pressures up to 30 MPa. And proposed a method for producing the catalyst.
DESCRIPTION OF THE INVENTION
The invention relates to chemical technology, particularly to methods for producing hydrogen by catalytic conversion of carbon monoxide, but also to compositions and methods for preparing catalysts for this process.
A method of producing hydrogen by steam reforming of carbon monoxide in the presence of 4-STC catalyst Pt-group, the process temperature of 200-250 C, steam-gas ratio of 0.4-0.8, a pressure of 30 MPa. (Azotchika Directory. M. Chemistry, 1986, pp. 144-146).
The disadvantages of this method are the limited capacity of the catalyst operating temperature regime and the impossibility of operating at temperatures up to 400 ° C, and the duration and reduction of the catalyst activation process up to 5-7 days and limiting the supply of reducing agent in the activation period of about 1-2. hydrogen, inability to carry out the process in one step.
Known method of preparing the catalyst for the conversion of carbon monoxide on the basis of carbonates of copper, zinc and aluminum hydroxide.
A disadvantage of the method is that in the process of mixing the components copper and zinc do not react, then reducing the catalyst activity. Furthermore, due to the increased speed of mass curing process of molding is complicated.
The task of the invention to provide a method for producing hydrogen by catalytic conversion of carbon monoxide in the low temperature mode, the cheap catalyst characterized by increased activity.
For this method of producing hydrogen by steam reforming is carried out carbon monoxide in the presence of a catalyst comprising, by weight. copper oxide 30-65; zinc oxide, 18-31; manganese dioxide 0,1-2,7; 0,08-0,8 carbon and a carrier based on alumina rest. In this conversion process is carried out at 190-400 ° C and pressures up to 30 MPa.
For steam reforming of carbon monoxide by forming hydrogen isomorphous mixture of copper and zinc salts in the feed solution on mixing with activated carbon and manganese compounds, after which the precipitate was separated and mixed with a carrier, then injected into the resulting mass Cu-Zn-Al or Cu- Zn-Al-Mn alloy in an amount of 1-40 wt. alloy components at a ratio of Cu-Zn-Al (1-6)  0.3-6)
0.3-6)  1-6) or Cu-Zn-Al-Mn (1-6)
1-6) or Cu-Zn-Al-Mn (1-6)  0.3-6): (1-6): (0.1-1), respectively, and intensive mixing is carried out until mass change its color to brown or gray-greenish-brown appearance and odor of alcohols, wherein the carrier is used as oxide aluminum or shungite or calcium aluminate.
0.3-6): (1-6): (0.1-1), respectively, and intensive mixing is carried out until mass change its color to brown or gray-greenish-brown appearance and odor of alcohols, wherein the carrier is used as oxide aluminum or shungite or calcium aluminate.
Using the above catalyst composition in the process of producing hydrogen can increase the degree of conversion at low temperature and to carry out the process in a single step process.
SUMMARY catalyst synthesis process consists in carrying out a solid phase reaction of starting components a mixture with alloys such alloy Devarda (Cu-Zn-Al and Cu-Zn-Al-Mn) with separation during intensive mixing of active atomic hydrogen that activates the starting state of the copper and allows the final eventually increase the catalyst activity while in service.
Introduced in the carbon composition has reducing properties and maintains an active state during the entire period of copper from the catalyst synthesis to manual process.
Manganese compound by changing the valency from 2 to 8 allow a transfer of oxygenate components form the metal salt to metal.
EXAMPLES EXAMPLE 1 (preparation of catalyst). The reactor was heated with intensive mixing, equipped with feed carbon dioxide and water, a mixture of isomorphic carbonate salts of copper and zinc in solution was added activated carbon and manganese oxide (the number of components, see Table 1). Then the precipitate formed is separated, it is dried and mixed with a carrier (carrier number and type cm. In the table).
The resulting mass is charged into a ball mill is filled to the same alloy Cu-Zn-Al in an amount of 1 wt. (Ratio of components in the alloy cm. In the table).
For forming a lubricant is introduced (copper stearate or graphite) in an amount of 1.5 wt. Was stirred in a ball mill until the alcohol odor and discoloration. Then the mass is discharged, humidified and molded into pellets. The resulting catalyst has the composition: Oxide Cu 40,2; oxide Zn 30,1; 0.8 carbon; media (Al 2 O 3) 28.1.
EXAMPLE Example s 2-6. The process is conducted as in Example 1 under the conditions reflected in Table 1.
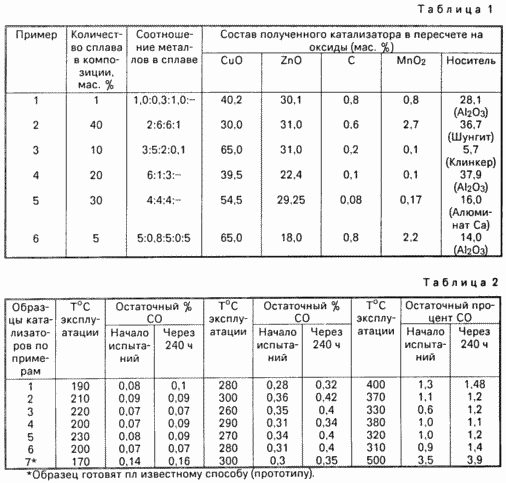
Examples for hydrogen. The catalyst after the recovery process is operated in an industrial environment in the process of conversion of carbon monoxide to hydrogen in the low temperature mode at 190 ° C with a ratio of steam: gas is 0.2, a space velocity of 3000 hr -1, a pressure of 2.8 MPa, a carbon monoxide 4.0 ± 1,0 vol. At the beginning of the test the residual content of carbon monoxide is about 0.09. after 240 hours of testing 0.1 vol. catalyst transferred to medium temperature operation test mode with pairs of expression: Gas 1.0 Operating temperature of 280 ° C with a content in the gas is 14,0 ± 3,0 vol. CO. The residual content of carbon monoxide in the beginning of the test is about 0,28-03,2. after 240 hours on test 0,36-0,4. the catalyst is transferred to operation mode at 400 ° C, a residual carbon monoxide content in the beginning of the test is 1.3. after 240 hours on test 1.48.
Testing of successive samples of Examples 1-6 are summarized in Table 2.
The proposed method allows a produce hydrogen conversion effective at low temperatures due to the high activity of the catalyst obtained in the latter without expensive noble metals.
CLAIM
1. A method of producing hydrogen by steam reforming of carbon monoxide in the presence of copper oxide-zinc-manganese catalyst, characterized in that the conversion is carried out in the presence of a catalyst comprising, by weight.
Copper oxide 30 65
Zinc Oxide 18 31
Manganese dioxide 0.1 2.7
Carbon 0.08 0.8
The carrier based on alumina rest
and the process is carried out at 190 400 o C and pressures up to 30 MPa.
2. A method for producing a catalyst for vapor conversion of hydrogen of carbon monoxide to hydrogen, comprising mixing copper and zinc compounds, followed by molding and curing the pellets, characterized in that a mixture isomorphic copper and zinc salts in a solution for mixing and additionally fed activated carbon and the compound manganese, whereupon the precipitate was separated and mixed with a carrier, then injected into the resulting mass Cu Zn Al- or Cu Zn Al Mn-alloy in an amount of 1 to 40 wt. at a ratio of alloy components Cu Zn Al June 1 0.3 6.0 1 6 or Cu Zn Al Mn 1 June 0.3 6.0 0.1 1.0 June 1, respectively, and carried out an intensive mixing of the masses to change its color to gray -burogo or greenish-brown and odor of alcohols.
3. A method according to claim 2, characterized in that used as a carrier of aluminum oxide or shungite or calcium aluminate.
print version
Publication date 26.02.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.