| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2285660
![]()
METHOD FOR PRODUCTION OF HYDROGEN AND METHANOL
The name of the inventor: Cherepnova Anna Viktorovna (UA); Lender Aida Anatolievna (UA); Pavlova Nadezhda Petrovna (UA); Kakichev Alexander Pavlovich (UA); Mitronov Alexander Petrovich (UA)
The name of the patent holder: State Research and Design Institute of Chemical Technologies "Chemotechnology" (UA)
Address for correspondence: 93400, Ukraine, Lugansk region, Severodonetsk, ul. Vilesova, 1, GNIiPI chemical technologies "Chemotechnology", OStNTIiPI, SS. Bykova
Date of commencement of the patent: 2004.04.29
The invention relates to a process for the production of technical hydrogen and methanol from a converted gas consisting essentially of CO, CO 2 , H 2 . The process for producing hydrogen and methanol from a converted gas containing carbon oxides and hydrogen involves the synthesis of methanol. The methanol synthesis is fed with a converted gas with a volume ratio of H 2 -CO 2 / CO + CO 2 of 2.03-5.4, which is carried out in a reactor system comprising a flow reactor or cascade of flow reactors and / or a gas mixture recycle reactor To obtain methanol, unreacted and purge gas. At the same time, a mixture of unreacted and converted gases is supplied for purification from carbon dioxide with its release and dosing of carbon dioxide into the converted gas fed to the synthesis of methanol. The purge gases are subjected to fine purification from impurities to produce hydrogen. The invention makes it possible to improve the process by maximizing the use of carbon oxides.
DESCRIPTION OF THE INVENTION
The invention relates to a process for the production of technical hydrogen and methanol from a converted gas consisting essentially of CO, CO 2 , H 2 .
A method for producing hydrogen obtained from synthesis gas by conversion of methanol with steam and a subsequent purification from carbon dioxide is known (US Patent No. 4,869,894, MKI C 01 B 1/13, filed April 15, 1987, published on September 26, 1989) .
The process proceeds according to the following main reactions:
Synthesis gas preparation process:
CH 4 + H 2 O = CO + 3H 2 -206.4 kJ / mol (1);
Process of conversion of carbon monoxide:
CO + H 2 O = CO 2 + H 2 +41.0 kJ / mol (2).
The disadvantage of this method is that during the production of hydrogen virtually all carbon of the synthesis gas is lost when carbon dioxide is washed off with monoethanolamine or potash from the gas mixture.
A method for producing hydrogen and methanol from a synthesis gas containing mainly carbon monoxide and hydrogen, including conversion of carbon monoxide, subsequent washing of carbon dioxide and synthesis of methanol, which is carried out before the conversion of carbon monoxide, is also known. For the synthesis of methanol, along with fresh synthesis gas, a recycle gas is used, the amount of recycle gas not exceeding twice the amount of fresh synthesis gas. The molar ratio of H 2 : CO in the gas fed to the synthesis is 0.8: 1-1.5: 1 (German Patent No. 2,904,008, MKI C 01 B 1/02, application 02.02.79, publ. 07.08 .80).
A disadvantage of the known method is that the composition of the gas is much lower than the stoichiometric composition; At such a ratio of reacting components methanol synthesis is characterized by a low rate and is accompanied by a large number of adverse reactions. Such a significant removal of the ratio of reacting components from stoichiometry results in a decrease in the conversion of carbon oxides to methanol due to a lack of hydrogen in the feed gas, and the residual CO 2 is blown out of the system.
Other drawbacks of this method include additional energy costs for compressing the flow to eliminate pressure losses inside the plant, to recirculate the unconverted gas mixture, but also that the return of a part of the product hydrogen leads to loss of the product.
The closest in technical essence is the method for producing methanol and hydrogen (PCT application No. 99/03807, application form 24.06.98, published on 28.01.1999, MKI C 07 C 31/04 - prototype), including
Steam reforming of the hydrocarbon feed at elevated pressure and temperature to form a gas stream containing hydrogen, carbon oxides, methane and unreacted steam;
- cooling of the produced gas with the release of condensed water;
- conversion of dry converted gas (without compression) into methanol and recovery of synthesized methanol;
- release of hydrogen from unreacted gas.
The unreacted gas may be subjected to a steam reforming prior to the evolution of hydrogen. In this case, the water vapor is dosed to a part of the unreacted gas and the steam-gas mixture undergoes a CO conversion, and the remainder is used as the cooling gas for conversion.
The synthesis of methanol can be carried out in one or more steps, with the isolation of synthesized methanol after each step, with methanol being separated from the reaction gas by washing with cold water.
The disadvantages of this known method of producing hydrogen and methanol include the following.
According to the description and the claims of PCT No. 99/03807 for the co-production of methanol and hydrogen, the converted gas obtained by steam reforming is used in a narrow range of the volume ratio H 2 -CO 2 / CO + CO 2 = 2.80-2.94. Thus, the applicability of this invention is limited, since the feedstock base is restricted by the use of converted gas obtained exclusively by steam reforming and does not apply to the use of converted gas obtained by other known conversion methods.
Another disadvantage is that the synthesis of methanol is conducted at a low pressure of 24-34 bar abs. It is known that during the synthesis of methanol in proportion to the decrease in pressure, the degree of conversion of carbon oxides decreases, the rate of formation and the yield of methanol decrease. [Technology of synthetic methanol: Sat. Naukch.tr., M., NIITEKHIM, 1989, p.74].
And, the lower the pressure, the greater difficulties arise when methanol is separated, since after the separator more methanol is carried off in the gas phase. In addition, a decrease in pressure contributes to the formation of saturated hydrocarbons - solid paraffins, the deposition of which hinders the work of heat exchangers [Synthetic methanol technology / Ed. Karavaeva M.M. - M .: Chemistry, 1984, p.102-103]. It is for these reasons that the process of synthesis of methanol on a copper-containing catalyst in the range of pressures of 50-100 atm is realized in industry.
The use of low pressure in the synthesis of methanol 24-34 bar abs. Leads the authors of the invention of PCT No. 99/03807 to the need to use a washing column with cold water irrigation to extract methanol, which will undoubtedly increase the energy costs for dehydration or rectification of raw methanol. Another negative effect of the use of reduced pressure is the low raw material production in methanol synthesis, and as a result, a large amount of waste gases from short-cycle adsorption (CCA). In the examples given, the quantity of waste gases ,. Containing a total of 70-81% by volume hydrogen and carbon oxides, is 25-30% of the total amount of converted gas sent to the joint production of hydrogen and methanol.
The object of the invention is to improve the process for the production of hydrogen and methanol, in which, by carrying out a process for producing methanol at a volume ratio (H 2 -CO 2 / CO + CO 2 ) of 2.03-5.4 and the organization of the process scheme Maximum processing of carbon oxides in the methanol synthesis section, and hydrogen is obtained from the purge gas after purification.
This object is achieved by the fact that in a process for producing hydrogen and methanol from a converted gas containing carbon oxides and hydrogen comprising methanol synthesis according to the invention, a converted gas with a volume ratio H 2 -CO 2 / CO + CO 2 equal to 2.03-5.4, which is carried out in a reactor system comprising a flow reactor or cascade of flow reactors and / or a gas mixture recycle reactor, to produce methanol, unreacted gas and purge gas, the mixture of unreacted and converted gases being fed for purification From carbon dioxide with its release and dosing of carbon dioxide into the converted gas fed to the synthesis of methanol.
The purge gas is subjected to a fine purification from impurities to produce hydrogen.
The carbon dioxide fed to the gas to be converted to methanol synthesis is metered out in the gas purification step, which is unreacted in the synthesis of methanol.
A distinctive feature of the claimed process for producing hydrogen and methanol is the methanol synthesis process at a volume ratio of H 2 -CO 2 / CO + CO 2 of 2.03-5.40 when the converted gas is fed to a reactor system comprising a flow reactor or a cascade of flow Reactors and / or a reactor with a recycle of the gas mixture, while maximizing the amount of carbon oxides. The purge gas after the synthesis reactor is immediately used as the product hydrogen. At the same time, the technological scheme is simplified by excluding from it the stages of conversion of carbon monoxide and purification from carbon dioxide, and the emission of carbon oxides into the atmosphere is completely absent in contrast to the prototype.
If only part of the total converted gas stream is taken for the synthesis of methanol, another part of this stream, mixed with unreacted gas, is sent to the carbon monoxide conversion compartment. The combined stream after conversion of CO is fed to absorption purification from carbon dioxide and product hydrogen is obtained.
Returning the carbon dioxide after the absorption purification step to the fresh converted gas stream fed to the methanol synthesis reactor, a volume ratio of H 2 -CO 2 / CO + CO 2 to stoichiometric ratio is adjusted to 2.03. In this case, the productivity of methanol is significantly increased, and the emission of carbon dioxide is reduced.
Studies have established that the advantages of joint production derive from the chemistry of interdependent reactions that describe the processes of obtaining hydrogen and methanol.
As it was said above, technically pure hydrogen is produced during the steam reforming of methane and other hydrocarbons, followed by the conversion of carbon monoxide by water according to the reactions (1), (2) and removal of carbon dioxide into the atmosphere from the gas mixture by solutions of potash and monoethanolamine.
In the joint production of hydrogen and methanol, carbon oxides serve as raw materials for the production of methanol in accordance with the following reactions:
CO 2 + 3H 2 -CH 3 OH + H 2 O + 49.53 KJ / mol (3);
CO + 2H 2 = CH 3 OH + 90.73 KJ / mol (4).
Thus, the carbon of natural gas is not released into the atmosphere, but is used to obtain a scarce, expensive product-methanol, which increases the profitability of the entire production in general. In addition, during the synthesis of methanol, product hydrogen is purified from carbon oxides.
The limit of the volume ratio H 2 -CO 2 / CO + CO 2 for the initial converted gas in the range from 2.03 to 5.4 is selected from the following considerations. Reduction of H 2 -CO 2 / CO + CO 2 is below 2.03, i.е. Below stoichiometric, leads to a decrease in the production of product hydrogen, to a decrease in the degree of conversion of carbon oxides to methanol, and, consequently, to additional costs to ensure the required degree of purification. In addition, the synthesis of methanol is characterized by a large number of adverse reactions, which deteriorates the quality of raw methanol. The upper limit for the volume ratio of H 2 -CO 2 / CO + CO 2 equal to 5.40 is determined by the fact that at a higher value of it due to a lack of carbon oxides in the converted gas, the specific productivity of the methanol catalyst decreases, which leads to an increase in the dimensions Methanol synthesis equipment.
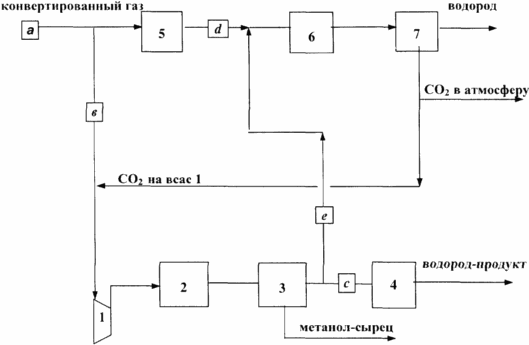
The proposed method for producing hydrogen and methanol is explained by the scheme shown in the figure, where
1 - the compressor;
2 - reactor system for the synthesis of methanol;
3 - gas cooling system and methanol extraction;
4 - fine purification of purge gas;
5 - high-temperature conversion of carbon monoxide;
6 - low-temperature conversion of carbon monoxide;
7 - purification from carbon dioxide;
A, c, c, d, e, - gas flows.
THE PROPOSED METHOD IS CONDUCTED AS FOLLOWS:
The converted gas from the conversion section (stream a), obtained by converting natural gas (steam, or steam or oxygen, or two-stage), is supplied to the compressor's intake position 1 and after compression to methanol synthesis. The reactor system of the methanol synthesis step 2 is a flow reactor or cascade of flow reactors and / or a gas recycle reactor. If all of the converted gas stream is fed to the synthesis of methanol (stream a is identical to stream c), the purge gas leaving the gas cooling system and the methanol recovery of item 3 (stream c) is sent to fine purification of pos. 4. Thus, from the scheme Co-production of methanol and hydrogen, the stages of high-temperature posture 5, low-temperature conversion of carbon monoxide pos.6 and adsorption purification from carbon dioxide are excluded.
In the case where it is necessary to change the ratio of the plant's powers towards increasing the power in hydrogen, the process is carried out as follows. Only part of the converted gas (stream in less than stream a) is taken out for methanol synthesis, and the remainder of the converted gas (stream d) is sent to the high-temperature conversion stage of CO, pos.5. The unreacted gas (stream c) after the methanol recovery system 3 is combined with the effluent leaving the high-temperature conversion (stream d) and fed to the low-temperature CO conversion of item 6, then to the carbon dioxide purification of item 7. The proposed method allows to vary the synthesis gas composition by supplying a part of carbon dioxide after purification of pos. 7 to the compressor suction in pos. 1, while reducing the emission of carbon dioxide into the atmosphere and accordingly increasing the degree of utilization of the carbon component of the feed.
Proof of the proposed method are the following examples.
Example 1 (comparative).
887 kmol / h of natural gas was supplied to the unit for producing the converted gas used for the synthesis of hydrogen and methanol at a pressure of 41 bar abs. After mixing with the recycled hydrogen and heating in the coil of the convection zone of the furnace, the mixed gas is fed to desulfurization. In natural gas purified from sulfur compounds, 2412 kmoles / h of superheated steam are dosed, the gas-vapor mixture is reheated to 540 ° C and sent to the reaction tubes of steam reforming. 4411 kmol / h of wet converted gas with a temperature of 865 ° C. of 37 bar are obtained. After cooling to 40 ° C and condensation of water, the converted gas in an amount of 2839 kmol / h is heated in a recuperative heat exchanger to 220 ° C and fed sequentially to two methanol synthesis reactors operating in flow mode. The synthesis of methanol takes place at a pressure of 34-32 bar at a temperature of 220-251 ° C. To regulate the temperature conditions in the reactors, cold bypass gas is supplied between the catalyst beds. After each synthesis reactor, raw methanol is separated from the reaction gas in a wash column, irrigating the gas with cold water. In this case, 221 and 178 kmol / h of methanol are produced, respectively, after the 1 st and 2 nd synthesis reactor, and in total 253 kmol / h of 100% methanol. Unreacted gas at a pressure of 31 bar in an amount of 2034 kmol / h is sent to the CCA and 1286 kmol / h of hydrogen is obtained. The results of the experiments are shown in the table.
Example 2.
The converted gas at a pressure of 8.0 MPa with a volume ratio of H 2 -CO 2 / CO + CO 2 equal to 5.40 is fed to a system of two flow reactors for the synthesis of methanol. The synthesis is carried out at a pressure of 5.0-5.5 MPa and a temperature of 200-260 ° C on a copper-zinc-aluminum catalyst. The purge gas after the release of methanol is given to the consumer as the product hydrogen. If it is necessary to reduce the content of impurities in the product hydrogen, methanol is condensed at reduced temperatures, using recycled water with a lower temperature or another refrigerant. The test results are given in the table.
Example 3.
Tests were carried out as in Example 2, but a converted gas with a volume ratio of H 2 -CO 2 / CO + CO 2 of 2.97 was fed to the synthesis of methanol and the process was carried out in a reactor with recycle of the gas mixture. In order to achieve the required hydrogen / methanol productivity ratio, the converted gas supplied to the synthesis reactor is approximately 43% of the total converted gas stream supplied to the plant. The rest of the stream (57%), bypassing the synthesis of methanol, is fed to the high-temperature conversion of carbon monoxide. After the methanol is separated, the unreacted gas from the synthesis section is combined with the converted gas stream (57%) before the low-temperature carbon monoxide conversion, after which the gas is supplied for absorption purification from CO 2 and product hydrogen is obtained. The test results are given in the table.
Example 4.
The tests are carried out as in Example 3, but 900 nm 3 / h of carbon dioxide is returned after the absorption carbon dioxide removal step into the fresh converted gas stream fed to the methanol synthesis reactor. The volume ratio of H 2 -CO 2 / CO + CO 2 is adjusted to a stoichiometric value of 2.0.3. In this case, the productivity of methanol is significantly increased, and the emission of carbon oxides decreases. The test results are given in the table.
Example 5.
The tests are carried out as in Example 2, but a converted gas with a volume ratio of H 2 -CO 2 / CO + CO 2 of 2.43 is fed to methanol synthesis, and methanol synthesis is carried out sequentially in the flow reactor and the recycle reactor. In this case, all converted gas supplied to the unit is sent to the methanol synthesis section. Purge gas after the reactor with a recycle with a hydrogen content of 86.43% is either given to the consumer as product hydrogen, or sent to a fine purification of impurities. The test results are given in the table.
As can be seen from the examples, the operation of the unit on the converted gas at a volume ratio of H 2 -CO 2 / CO + CO 2 equal to 2.03-5.40 makes it possible to increase the conversion of the sum of carbon oxides from 41.85% achieved in the prototype, To 93.68-96.23% in the proposed method. At the same time, the gas emissions are either completely eliminated or the amount of exhaust gases from 250 nm 3 per 1000 nm 3 of converted gas in the prototype is reduced to 38-124 nm 3 per 1000 nm 3 of the converted gas in the proposed method. On the basis of the foregoing, it can be concluded that the proposed method for producing hydrogen and methanol allows for a flexible variation of the ratio of capacities for hydrogen and methanol, based on market demand, more efficient use of raw materials, significantly reducing the loss of its carbon component and improving the environmental performance of the process by reducing emissions of carbon oxides in Atmosphere.
| № п / п | The name is indicative | Units | Example 1 | Example 2 | Example 3 | Example 4 | Example 5 | Example 6 |
| 1 | 2 | 3 | 4 | 5 | 6th | 7th | 8 | 9 |
| 1 | Converted gas to a hydrogen and methanol production unit: flow rate | Nm 3 / h | 63594 | 150000 | 30857 | 30857 | 70000 | 112192 |
| Composition | % Vol. | |||||||
| H 2 | 71.04 | 84.0 | 75.94 | 75.94 | 67.39 | 71.73 | ||
| CO | 13.07 | 12.0 | 10.29 | 10.29 | 25.54 | 20.57 | ||
| CO 2 | 8.31 | 3.0 | 11.4 | 11.4 | 4.95 | 6.32 | ||
| N 2 | 0.21 | 0.5 | 0.37 | 0.37 | 1.16 | 0.48 | ||
| CH 4 | 7.12 | 0.5 | 2.00 | 2.00 | 0.96 | 0.90 | ||
| H 2 O | 0.25 | |||||||
| Quantity of CO + CO 2 | Nm 3 / h | 13596 | 22500 | 6693 | 6693 | 21343 | 30168 | |
| 2 | Synthesis of methanol in stages | |||||||
| Fusion pressure | MPa | 3.4 | 8.0 | 5.0 | 5.0 | 5.3 | 6.0 | |
| Type of reactor used | Flowing | Flowing | - | - | - | Flowing | ||
| Number of reactors | 2 | 2 | 1 | |||||
| - | - | Recycled | Recycled | Recycled | Recycled | |||
| 1 | 1 | 1 | 1 | |||||
| The selection of the converted gas for the synthesis of methanol: | ||||||||
| Consumption | Nm 3 / h | 63594 | 150000 | 13250 | 13250 | 70000 | 112192 | |
| Share of total flow | % | 100 | 100 | 43 | 43 | 100 | 100 | |
| Dosage of CO 2 from the methanol synthesis purification section: flow rate | Nm 3 / h | 900 |
| Table continuation | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6th | 7th | 8 | 9 |
| Converted gas to | ||||||||
| Methanol synthesis: consumption | Nm 3 / h | 63594 | 150000 | 13250 | 14150 | 70000 | 112192 | |
| Composition H 2 | % Vol. | 71.04 | 84.0 | 75.94 | 71.11 | 67.39 | 71.73 | |
| CO | 13.07 | 120 | 10.29 | 9.64 | 25.54 | 20.57 | ||
| CO 2 | 8.31 | 3.0 | 11.40 | 17.03 | 4.95 | 6.32 | ||
| N 2 | 0.21 | 0.5 | 0.37 | 0.35 | 1.16 | 0.48 | ||
| CH 4 | 7.12 | 0.5 | 2.00 | 1.87 | 0.96 | 0.9 | ||
| H 2 O | 0.25 | |||||||
| Quantity of CO + CO 2 | Nm 3 / h | 13596 | 22500 | 2874 | 3773 | 21343 | 30168 | |
| The ratio of H 2 -CO 2 / CO + CO 2 | 2.80 | 5.40 | 2.97 | 2.03 | 2.05 | 2.43 | ||
| 2.1 | Synthesis of methanol in flow reactors | - | - | - | ||||
| 2.1.1 | Volume of catalyst | M 3 | Not specified in the prototype | 32 + 16 = 48 | 26.15 | |||
| 2.1.2 | I / O temperature. | FROM | 220/251 | 230/270 | 230/270 | |||
| 2.1.3 | Gas at the outlet of the cascade of flowing reactors: flow rate | Nm 3 / h | 48406 | 87805 | 89730 | |||
| Ingredients: CO 2 | % Vol. | 9.12 | 0.68 | 7.23 | ||||
| H 2 | 68.16 | 90.58 | 64.00 | |||||
| H O | 1.06 | 2.20 | 0.67 | |||||
| N 2 | 0.28 | 0.85 | 0.60 | |||||
| CH 4 | 9.34 | 0.85 | 1.13 | |||||
| CO | 7.13 | 0.35 | 13.87 | |||||
| CH 3 OH | 4.91 | 4.49 | 12.50 | |||||
| 2.1.4 | Total amount of water for washing methanol out of gas | Kg / hr | 1800 | There is no stage of washing methanol with water | ||||
| 2.1.5 | Methanol - raw, consumption | T / h | 11,742 | 15.66 | ||||
| 2.1.6 | 100% CH 3 OH | T / h | 8,128 | 30.11 | 15,113 | |||
| 2.1.7 | The degree of conversion of CO + CO 2 to methanol | % | 41.85 | 93.68 | - | - | - | 35.06 |
| Table continuation | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6th | 7th | 8 | 9 |
| 2.2 | Synthesis of methanol in a recycle reactor | - | - | |||||
| 2.2.1 | Volume of catalyst | M 3 | 15.5 | 15.5 | 61 | 61 | ||
| 2.2.2 | I / O temperature. | FROM | 210/246 | 235/269 | 240/261 | |||
| 2.2.3 | Fresh gas: consumption | Nm 3 / h | 13250 | 14150 | 70000 | 77470 | ||
| Ingredients: CO 2 | % Vol. | 11.40 | 17.03 | 4.95 | 8.23 | |||
| H 2 | 75.94 | 71.11 | 67.39 | 73.27 | ||||
| BUT | - | - | - | 0.01 | ||||
| N 2 | 0.37 | 0.35 | 1.16 | 0.69 | ||||
| CH 4 | 2.00 | 1.87 | 0.96 | 1.27 | ||||
| CO | 10.29 | 9.64 | 25.54 | 15.77 | ||||
| CH 3 OH | - | - | - | 0.76 | ||||
| 2.2.4 | Gas inlet / outlet in | Nm 3 / h | 130000 | 139423 | 600,000 | 600,000 | ||
| Reactor: flow rate | %about. | 124485 | 132154 | 559535 | 563915 | |||
| Ingredients: CO 2 | 2.08 / 1.03 | 7.18 / 5.84 | 4.30 / 4.10 | 2.62 / 1.73 | ||||
| H 2 | 85.92 / 84.15 | 58.68 / 54.68 | 61.74 / 58.44 | 84.64 / 82.59 | ||||
| H 2 O | 0.14 / 1.29 | 0.21 / 1.95 | 0.07 / 0.60 | 0.11 / 1.18 | ||||
| N 2 | 2.12 / 2.22 | 7.47 / 7.90 | 12.18 / 13.06 | 2.89 / 3.08 | ||||
| CH 4 | - | 7.21 / 7.53 | 22.70 / 23.94 | 9.69 / 10.40 | 5.08 / 5.40 | |||
| CO | 1.90 / 0.92 | 2.91 / 2.05 | 11.24 / 8.96 | 3.93 / 2.08 | ||||
| CH 3 OH | 0.64 / 2.87 | 0.85 / 3.64 | 0.78 / 4.44 | 0.73 / 3.94 | ||||
| 2.2.5 | Methanol raw | T / h | 5,044 | 7.146 | 31,684 | 31,211 | ||
| 100% methanol | 3.891 | 5,185 | 28,847 | 26,203 | ||||
| 2.2.6 | Total | T / h | ||||||
| Of methanol produced: - crude | 11,742 | 5,044 | 7.147 | 31,684 | 46.87 | |||
| -100% CH 3 OH | 8,128 | 30.11 | 3.891 | 5,185 | 28,847 | 41.32 | ||
| 2.2.7 | Total degree | % | ||||||
| Conversion of CO + CO 2 into | 41.85 | 93.68 | 96.23 | 95.97 | 94.61 | 95.86 | ||
| Methanol in the synthesis compartment | ||||||||
| Table continuation | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6th | 7th | 8 | 9 |
| 2.2.8 | Unreacted gas from | |||||||
| Synthesis of methanol on | ||||||||
| Production of hydrogen: consumption | Nm 3 / h | 45562 | 81670 | 3643 | 873 | 5976 | 17261 | |
| Ingredients: CO 2 | % Vol. | 9.63 | 0.72 | 1.10 | 6.07 | 4.22 | 1.78 | |
| H 2 | 72.43 | 96.67 | 87.49 | 57.28 | 61.00 | 86.32 | ||
| H 2 O | 0.10 | 0.05 | 0.20 | 0.23 | 0.08 | 0.13 | ||
| N 2 | 0.29 | 0.51 | 2.10 | 8.28 | 13.63 | 3.21 | ||
| CH 4 | 9.93 | 0.89 | 7.40 | 25.06 | 10.85 | 5.64 | ||
| CO | 7.57 | 0.87 | 1.00 | 2.15 | 9.35 | 2.17 | ||
| CH 3 OH | 0.05 | 0.38 | 0.71 | 0.93 | 0.87 | 0.75 | ||
| 3 | Gas cleaning | |||||||
| 3.1 | Conversion of CO | |||||||
| 3.1.1 | Source selection. Gas at | |||||||
| Conversion of carbon monoxide | ||||||||
| Before synthesis of methanol: consumption | ||||||||
| (In terms of dry gas) | Nm 3 / h | - | - | 17607 | 17607 | - | - | |
| Humid | 33109 | 33109 | ||||||
| 3.1.2 | Pressure | MPa | 21.5 | 21.4 | ||||
| 3.1.3 | I / O temperature | FROM | ||||||
| High-temperature | ||||||||
| Conversion | 345/390 | 345/390 | ||||||
| Low-temperature | ||||||||
| Conversion | 208/224 | 220/233 | ||||||
| 3.1.3 | Gas output from conversion | |||||||
| Carbon monoxide after | ||||||||
| Department of process | ||||||||
| Condensate: consumption | Nm 3 / h | - | 22807 | 20020 | - | - | ||
| Ingredients: CO 2 | % Vol. | 17.08 | 18.97 | |||||
| H 2 | 79.59 | 77.74 | ||||||
| N 2 | 0.71 | 0.76 | ||||||
| CH 4 | 2.46 | 2.38 | ||||||
| CO | 0.16 | 0,15 | ||||||
| Table continuation | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6th | 7th | 8 | 9 | |
| 3.2. | Carbon Dioxide Purification | ||||||||
| 3.3. | Type of cleaning from СО 2 | - | Adsorption | Adsorption | - | - | |||
| 3.2.1 | The amount of CO 2 liberated and the atmosphere | Nm 3 / h | 3895 | 3798 | |||||
| 4 | Thin hydrogen purification from impurities | ||||||||
| 4.1 | Fine cleaning | CCA | Separation of methanol during slaking (t = 0 ° C) | - | - | CCA | |||
| 5 | Product hydrogen after | ||||||||
| Cleaning: consumption | Nm 3 / h | 28806 | 81238 | 18912 | 16222 | 3280 | 17261 | ||
| Ingredients: CO 2 | % Vol. | 0.70 | Sl. | Sl. | 1.78 | ||||
| H 2 | 100 | 97.08 | 95.5 | 95.96 | 100 | 86.32 | |||
| H 2 O | - | - | - | 0.13 | |||||
| N 2 | 0.91 | 0.9 | 0.93 | 3.21 | |||||
| CH 4 | 0.89 | 3.4 | 2.93 | 5.64 | |||||
| CO | 0.37 | 0.2 | 0.18 | 2.17 | |||||
| CH 3 OH | 0.06 | 0.75 | |||||||
| 6. Overall indicators | |||||||||
| 6.1. | Initial converted gas: total | Nm 3 / h | 63594 | 150000 | 30857 | 30857 | 70000 | 112192 | |
| The functional H 2 -CO 2 / CO + CO 2 | 2.80 | 5.40 | 2.97 | 2.03 | 2.05 | 2.43 | |||
| 6.2. | Number of hydrogen produced | Nm 3 / h | 28806 | 81238 | 18912 | 16222 | 3280 | 17261 | |
| T / h | 2,572 | 10,090 | 2,299 | 1,870 | 0.295 | 4,000 | |||
| 6.3. | Number of received 100% - | Nm 3 / h | 5690 | 21077 | 2724 | 3630 | 20193 | 28924 | |
| Methanol | T / h | 8,128 | 30.11 | 3.891 | 5,185 | 28,847 | 41.32 | ||
| 6.4. | The ratio H 2 / CH 3 OH: | ||||||||
| Massive | T / t | 0.316 | 0.335 | 0.591 | 0.361 | 0.010 | 0.097 | ||
| Three-dimensional | Nm 3 / nm 3 | 5,063 | 3.854 | 6,943 | 4,469 | 0.162 | 0.596 | ||
| Table continuation | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6th | 7th | 8 | 9 |
| 6.5. | The total conversion of CO + CO 2 to methanol in the synthesis compartment | % | 41.85 | 93.68 | 96.23 | 95.97 | 94.61 | 95.86 |
| 6.6 | Discharge gases in the joint production of hydrogen and methanol: the amount | Nm 3 per 1000 nm 3 kon. Of gas | 250 | 0 | 124 | 121 | 38 | 0 |
| composition: | % Vol. | |||||||
| CO 2 | 27.68 | 100 | 100 | 9.35 | ||||
| CO | 21.75 | 20.73 | ||||||
| H 2 | 20.76 | 13.42 | ||||||
| CH 4 | 28.54 | 24.04 | ||||||
| N 2 | 0.85 | 30.34 | ||||||
| H 2 O | 0.28 | 0.19 | ||||||
| CH 3 OH | 0.14 | 1.93 | ||||||
CLAIM
A method for producing hydrogen and methanol from a converted gas containing carbon oxides and hydrogen comprising methanol synthesis, characterized in that a converted gas with a volume ratio of H 2 -CO 2 / CO + CO 2 of 2.03- 5.4, which is carried out in a reactor system comprising a flow reactor or cascade of flow reactors and / or a gas mixture recycle reactor to produce methanol, unreacted gas and purge gas, the mixture of unreacted and converted gases being fed to the carbon dioxide Release and dosing of carbon dioxide into the converted gas fed to methanol synthesis.
2. A method according to claim 1, characterized in that the purge gases are subjected to a fine purification from impurities to produce hydrogen.
print version
Publication date 27.02.2007gg




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.