| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2031834
![]()
HYDROORAGING MATERIAL FOR THE PRODUCTION OF HYDROGEN AND THE METHOD OF ITS OBTAINING
The name of the inventor: Kirillov Vladimir Ivanovich ; Yastrebov Alexander Nikolaevich
The name of the patent holder: Kirillov Vladimir Ivanovich ; Yastrebov Alexander Nikolaevich
Address for correspondence:
Date of commencement of the patent: 1990.06.07
Application: hydrogen-oxygen fuel cells, internal combustion engines, gas generators for hand devices, etc. Essence: the material for hydrogen production contains components at the following ratio, wt. %: Zinc 0.5-0.02; The catalyst is 0.5 to 3.0. Nickel and / or cobalt and / or manganese are used as a catalyst. The material is prepared as follows: magnesium is heated to the melting point and catalyst and zinc are added thereto. The melt is mixed and poured. The casting is cooled at a rate of 0.1 - 2.0 deg / s. The concentration of the catalyst in the alloy is determined depending on the required rate of hydrogen evolution according to the formula: v = 14 c · e 0.0036t , where v is the hydrogen evolution rate, cm 3 / cm 2 s is the catalyst concentration in the alloy, %; E is the base of the natural logarithm; T - temperature in the reaction zone of hydrogen formation, 14 - coefficient.
DESCRIPTION OF THE INVENTION
The invention relates to the field of hydrogen energy, in particular, to metallic compounds that release hydrogen when interacting with water, and is used in hydrogen-oxygen fuel cells, internal combustion engines, hydrogen blowing pods, gas generators for apparatuses for cutting, welding and soldering metals, plasma chemistry for Creation of a reducing gas environment, etc.
There are various technologies for producing hydrogen, for example, by electrolysis of water, processing of coal with the help of known water-gas reactions, treatment of natural gases and from metallic compounds.
A class of metal compounds known as hydrides and which are sources of hydrogen-hydrides of alkaline, alkaline-earth metals and their alloys, Group III metals and their alloys, is known. [1]. Hydrides under certain conditions absorb hydrogen, and at others - it is isolated.
The above sources of hydrogen are expensive (expensive metals used in them, a sophisticated technology for obtaining both the hydrides themselves and hydrogen from them). In addition, the reactions of hydrogen production from hydrides are inert, since hydrides are chemically unstable.
Magnesium is the cheapest of the above metals, so the production of hydrogen from its hydrides has found wide application.
Known, for example, is a composition for the accumulation of hydrogen based on magnesium, containing 20-30 wt% nickel and an additional 0.15-1.5 wt% silicon to increase its sorption capacity [2].
Disadvantages of this composition are its low corrosion resistance, since magnesium is very active in the interaction with water, and low efficiency due to the high content of nickel. Such formulations require special storage conditions.
There are known materials for the production of hydrogen by their interaction with water, called hydro-reactive.
Such materials include, for example, aluminum-based materials containing various metals as catalyst.
The object is achieved by the fact that the hydro-reactive material for hydrogen production contains zinc, catalyst and magnesium at the following component ratio, by weight: Zinc 0.05-0.02 Catalyst 0.5-3.0 Magnesium balance, the catalyst being nickel and Cobalt and / or manganese.
The aim is achieved by the fact that in the production of a hydro-reactive material, including the heating of the base of the material, the introduction of a catalyst into it, the casting of the material and its cooling, magnesium is used as the base, which is heated to the melting point, the catalyst and passivator are introduced into the melt, Of the resulting alloy is cooled at a rate of 0.1-2.0 deg / s. In this case, the concentration of the catalyst additives is determined depending on the desired rate of hydrogen evolution according to the following formula: ![]() Where C is the concentration of the catalyst in the alloy, mass%;
Where C is the concentration of the catalyst in the alloy, mass%;
V is the hydrogen evolution rate, cm 3 / cm 2 ;
L - the base of natural logarithms;
T - temperature in the reaction zone of hydrogen formation, о С;
14 - coefficient.
The results of experimental studies have shown that, depending on the amount of catalyst and passivator, the alloys exhibit slightly different properties, and the cooling rate of the casting from these alloys also has significance.
Example 1 . The alloy for rapid response, used, for example, to blow the submarine system. 20 kg of magnesium, for example, MG95, were placed in the induction furnace, melted it, 0.6 kg of nickel (3% by weight) and 0.05 kg of zinc, for example of the CO, were introduced in a solid form. The alloy was mixed with a mechanical stirrer, then cast and cast, cooled at a rate of 2 deg / s to 50 ° C, i.e., the casting was made into a water-cooled mold. The rate of hydrogen evolution during interaction with sea water is 100 m 3 / s.
Example 2 . A slow-response alloy used, for example, for sea buoy hydrostats. The composition of the alloy, wt.%: Nickel 0.5; Cobalt 0.2; Zinc 0.05. The casting of the alloy was cooled at a rate of 0.1 deg / s to 50 ° C. The structure of the alloy is less dense than in Example 1, since the cooling rate is less. The rate of hydrogen evolution during interaction with seawater is 10 m 3 / s.
Example 3 . Alloy for use, for example, in internal combustion engines. The composition of the alloy, wt.%: Nickel 1; Manganese 0.02; Zinc 0.03. In all examples, the temperature of the hydrogen evolution reaction is 100 ° C.
Example calculation. Let us assume that a hydrogen evolution rate of 10 cm / cm xx min is required at T = 100 ° C. Then the catalyst concentration is determined by the equation: ![]()
Thus, C - the required catalyst concentration for this gas evolution rate is:
C = 0.5% by weight
The remaining examples of alloys with different components are given in the table.
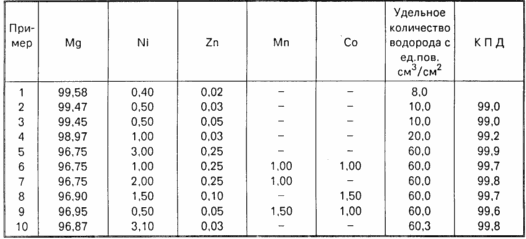
The results of the experiments show that the greatest value for the efficiency of hydrogen evolution is the amount of nickel in the alloy.
Thus, the proposed composition of the hydro-reactive material has a high corrosion resistance in air, while the efficiency of the hydrogen evolution process is 99.0-99.9%.
CLAIM
1. A hydro-reactive material for the production of hydrogen, comprising zinc, a catalyst and magnesium, characterized in that, in order to increase the corrosion resistance of the material in air and the hydrogen production, nickel and / or cobalt and / or manganese are used as a catalyst at the following component ratio, Wt%:
Zinc - 0.5 - 0.02
Catalyst 0.5 to 3.0
Magnesium - Other
2. A process for the production of a hydro-reactive material for the production of hydrogen, comprising heating a substance releasing hydrogen, introducing a catalyst into it, casting the material and cooling the casting, characterized in that, in order to increase the corrosion resistance of the material in air and the hydrogen productivity, , Magnesium is used, its heating is carried out to the melting point and zinc is added to the melt simultaneously with the catalyst, mixed, and cooling of the casting is carried out at a rate of 0.1-2.0 deg / s, the catalyst concentration in the alloy being determined depending on the desired release rate Hydrogen by the formula ![]()
Where V is the rate of hydrogen evolution, cm 3 / cm 2 ;
C is the catalyst concentration in the alloy, wt.%;
T is the temperature in the reaction zone of hydrogen formation;
E is the base of the natural logarithm;
14 - coefficient.
print version
Date of publication 29.02.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.