| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2285305
![]()
METHOD (VARIANTS) AND A DEVICE FOR THE ALLOCATION hydrogen isotopes (variants)
Name of the inventor: Takahiko Sugiyama (JP); Yamato Asakura (JP); UDD Tatsuhiko (JP); YAMAMOTO Ichiro (JP); ENOKIDA Ёuiti (JP)
The name of the patentee: Inter-University RESEARCH CORPORATION INSTITYUT National INSTITYUTS PF NEYCHURAL SAYNSES (JP)
Address for correspondence: 103735, Moscow, ul. Ilinka, 5/2 "Sojuzpatent" N.N.Vysotskoy
Starting date of the patent: 2004.09.09
The invention relates to producing hydrogen isotopes. The inventive method of separating hydrogen isotope comprises the steps of bringing a medium containing the hydrogen gas includes said hydrogen isotope, a water and water vapor in a reduced pressure condition of 90 kPa or lower. Controlling the temperature of said process medium in accordance with the pressure in said medium. The regulation of the partial pressures of hydrogen gas and steam and separation efficiency control said hydrogen isotope from a gaseous hydrogen exchange reaction using a hydrogen-water system. Device for hydrogen isotope separation includes separation column adapted to separate hydrogen isotope from a gaseous hydrogen flow as a result of the exchange reaction in the hydrogen-water system. The apparatus comprises a temperature controller and providing temperature control of the medium in accordance with its pressure regulation of the partial pressures of hydrogen gas and water vapor and efficiency of regulation of the evolution of hydrogen isotopes of hydrogen gas. The advantages of the invention are to improve the efficiency of selection.
DESCRIPTION OF THE INVENTION
The present invention relates to a process for separating hydrogen isotopes and a device for its release.
As a method for separation and concentration of tritium from tritiated water component provides a process which uses hydrogen exchange reaction in-water system, characterized by a high separation factor and capable of providing a large number of processing tritiated water. Since equilibrium constants of the isotope exchange reaction between hydrogen gas and water vapor between steam and water in the exchange reaction in the hydrogen-water system, increases with decreasing temperature of the exchange reaction, one can expect that the process temperature reduction will improve isolation tritium component.
However, at lower partial pressure reduced process temperature steam used in the exchange reaction, thereby reducing the effectiveness of selection the tritium component.
US Patent 5093098, 1992, described a method and apparatus for separation of hydrogen isotope, comprising monitoring the effectiveness of hydrogen isotope separation of hydrogen gas by using an exchange reaction to hydrogen-water system, carried in a separation column. Furthermore, the device comprises a temperature controller for registration of temperature increase in comparison with the working temperature. This invention has a number of drawbacks which are eliminated by the following present invention.
SUMMARY OF THE INVENTION
The purpose of the present invention is to improve the release of hydrogen isotope exchange reaction using a hydrogen-water system.
To achieve the above object, the present invention employs a method of separating a hydrogen isotope, comprising the steps of:
bringing a medium containing the hydrogen gas includes said hydrogen isotope, a water and water vapor, to specify the conditions of reduced pressure, and
controlling the process temperature in said medium in correspondence with the pressure in said medium in order to regulate the partial pressures of hydrogen gas and water vapor and efficiency of separation of said hydrogen isotope from a gaseous hydrogen exchange reaction using a hydrogen-water system.
In addition, the present invention relates to an apparatus for separation of hydrogen isotope, comprising:
a separation column through which the medium containing the hydrogen gas comprises a hydrogen isotope, a water and water vapor, which is released in said hydrogen isotope from the hydrogen gas by a hydrogen exchange reaction-water system, and
Device for the temperature control (temperature controller) designed to regulate the temperature of the medium in accordance with the pressure of the medium, adjusting the partial pressures of hydrogen gas and water vapor separation efficiency and adjusting said hydrogen isotope from the hydrogen gas.
To achieve this goal, the inventors had conducted extensive research. As a result, it was found that if in a medium containing hydrogen gas comprising the hydrogen isotope, a water and steam are supplied to the exchange reaction in the hydrogen-water are given low pressure conditions, the efficiency of separation of hydrogen isotope can be maximized at a certain temperature and specific pressure decreases. In this regard, if the medium is formed in the separation column, under certain conditions of reduced pressure and temperature controlled environment mode in accordance with the conditions of reduced pressure by means of temperature control, it can be achieved maximum efficiency of the separation of hydrogen isotopes of hydrogen gas.
The reduced pressure not necessarily maintain the temperature environment at the optimum value, but it is necessary to set the temperature corresponding to the desired separation efficiency of the hydrogen isotope.
According to a preferred embodiment of the present invention, Wednesday maintained at a pressure of 90 kPa or lower. In this case, the increased efficiency provided by the release of hydrogen isotope exchange reaction using a hydrogen-water system.
In accordance with another embodiment of the present invention, the ambient temperature increase is registered in the separation column in static separation using an exchange reaction to hydrogen-water system. In this case, the flow can be detected from the separation column, and can be detected before the leak of the entire device, including the separation column can be prevented whereby an unavoidable accident such as the explosion of hydrogen.
Besides hydrogen isotope separation method of the present invention in which a certain set environment temperature under reduced pressure, the above method of leak detection can be used for another process for separating hydrogen isotopes, which is used in the exchange reaction of hydrogen-water system.
As noted above, the present invention can provide improved separation efficiency of hydrogen isotope exchange reaction using a hydrogen-water system.
BRIEF DESCRIPTION OF DRAWINGS
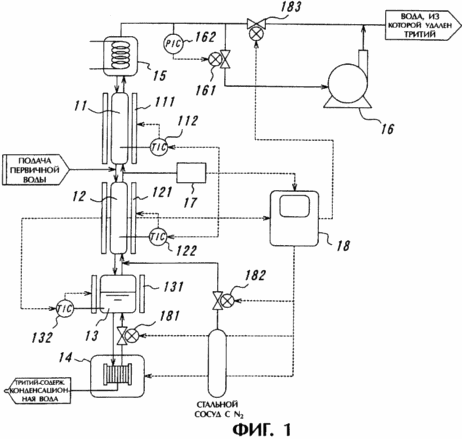
For a better understanding of the present invention should be had to the accompanying drawings, in which
1 shows a general view of an apparatus for separation of hydrogen isotope according to the present invention. In accordance with this embodiment of tritium released from the component by means of light water separating device depicted.
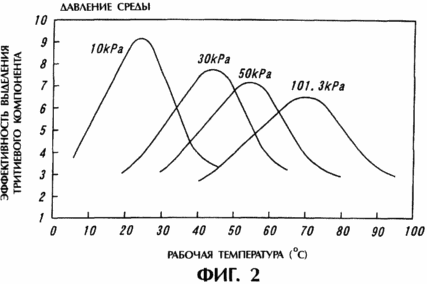
2 is a graph illustrating the dependence of efficiency of selection the tritium component on the pressure and temperature of the medium in the separation column in a process of implementing selection the tritium component.
The separation device shown in Figure 1, includes separation columns 11 and 12 arranged in the vertical two-step configuration, the humidifier 13, the temperature controller 132 and the bottom side of the separating columns SRE electrolyzer 14, refrigerator 15 and a vacuum pump 16 located above the separating columns , tritium monitor 17 equipped with a monitor and temperature controller 18 disposed at a level between 11 and 12 columns.
The separation columns 11 and 12 are formed catalyst layers (not shown) containing a platinum catalyst providing the first flow of the exchange reaction between hydrogen gas and water vapor, and the adsorbent layer (not shown) to ensure the flow of the second exchange reaction between water vapor and liquid.
On the periphery of the separating columns 11 and 12 are heaters 111 and 121, which by means of temperature controllers 112 and 122 provide the heating medium in the separating columns 11 and 12 to the operating temperature. Likewise, on the periphery of the humidifier 13 is a heater 131, which provides the formation of water vapor resulting from heating water.
Light water, representing the raw water containing the tritium component is fed into separation columns 11 and 12 and then inputted to the SPE electrolyzer 14 through the humidifier 13. The light 14 SPE electrolytic water containing the tritium component is subjected to electrolysis and converted into hydrogen gas and oxygen. Hydrogen gas tritium component comprises a hydrogen isotope. Hydrogen gas is introduced into the humidifier 13 is saturated with water vapor, and then fed into the separating columns 11 and 12.
The hydrogen gas and water vapor is partially received in the refrigerator 15, after passing through the separating columns 11 and 12 by a vacuum pump 16. Thereafter, steam is introduced into enrichment undergoes heat exchange in cooler 15 and returned as liquid water in separation columns 11 and 12. The output of hydrogen gas is provided by a vacuum pump 16 via a pressure controller 162 and safety valve 161.
According to this embodiment, on the catalyst layers in the separating columns 11 and 12 takes place following the first exchange reaction:
![]()
In addition, the absorbent layers in separation columns 11 and 12 following the second exchange reaction takes place:
![]()
In the second exchange reaction intermediate steam HTO (vapor) is fed countercurrently to the refrigerator 15 enriched in recycled liquid water and H 2 O (liquid). As a result, the isolation and enrichment of tritium component in the liquid reacted water HTO (liquid).
Liquid unreacted water flows downwardly through the separating columns 11 and 12 and 14 and SPE electrolyzer extracted outside the separation device. On the other hand, the hydrogen gas H 2 (gas), forming a first exchange reaction by separation HTO (vapor) flows upwardly through column 11 and separator 12 and exits the separation device.
2 is a graph illustrating the dependence of efficiency of selection the tritium component on the pressure and temperature of the working medium in the separating columns 11 and 12 under the tritium component separation process. From Figure 2, that the efficiency of separation of the tritium component exhibit various temperature dependencies, are in accordance with the pressure medium in the separating columns 11 and 12. From Figure 2 it follows that the optimum operating temperature for maximum separation efficiency of the tritium component differ from each other and correspond to certain values of the pressure medium in the separating columns 11 and 12.
In the separating apparatus shown in Figure 1, the tritium component is released, its concentration and excretion downcomer in the liquid reacted water HTO (liquid). In this connection, the concentration of the tritium component is low in the upper portions of the separating columns 11 and 12 and the bottom of the column is high. By increasing the efficiency of selection the tritium release characteristics of its components can be represented by the ratio of the tritium component concentrations in the upper and lower parts of the separating columns 11 and 12 (i.e., the concentration ratio of the tritium component at the bottom of the separating column / the concentration of the tritium component at the top of the separation column) .
Thus, the separation efficiency of the tritium component illustrated in Figure 2 can be indirectly determined from the ratio of its concentrations in the lower and upper parts of the separating columns 11 and 12 (the concentration of the tritium component at the bottom of the separating column / the concentration of the tritium component at the top of the separation column) .
According to this embodiment, the concentration ratio of the tritium component is controlled by tritium monitor 17. More specifically, supervised tritium at the top of the separation column 12, and the working environment temperature in the separation column 11 and 12 are regulated by a temperature control regulators 112 and 122 by the control of the temperature using the controller 18 and heaters 111 and 121, thereby minimizing the concentration of the tritium component at the top of the separation column 12 (i.e., the concentration of the tritium component at the bottom of separation column 12 is maximized for maximum separation efficiency of the tritium component).
As can be seen from Figure 2, the tritium component separation efficiency increases with decreasing pressure in the medium separating columns 11 and 12. For example, to implement practically acceptable efficiency of the tritium release component, the pressure medium should be maintained at or below 90 kPa. However, for component selection the tritium exchange reaction using hydrogen in the system using steam-atmospheric pressure of about 10 kPa.
The subject method can be used to provide the desired separation efficiency tritium component, but also to increase the efficiency at the design discharge pressure. In this case, the process temperature is controlled in such a way as to provide the desired separation efficiency tritium component.
On the other hand, during the isolation process using the tritium component separating apparatus shown in Figure 1, the medium located in the separating columns 11 and 12 under reduced pressure. In this connection, if found to flow from the separation column 11 and / or 12, the media temperature in separation columns 11 and / or 12 is substantially increased due to the chemical reaction between hydrogen and oxygen in the catalyst layers. Thus, the flow from the separating columns 11 and / or 12 may be detected by an increase in environmental temperature, compared to an operating value which is fixed by a temperature controller 18. Upon detection of a leak from the separating columns 11 and / or 12 ceases operation SPE electrolyzer 14 and off heating the separating columns 11 and 12. After that, the relief valve 181 is closed and open valves 182 and alarm 183, resulting in the substitution of internal fluids separation columns 11 and 12 and are purged with nitrogen gas.
Detection of leakage from the separating columns 11 and 12 using the temperature measuring controller 18 may be performed simultaneously or separately with the tritium component separating process in which the environment temperature and the temperature optimization process.
Although the present invention has been described in detail with reference to the examples of its embodiments, the invention is not so limited and includes any description of changes and modifications without violating the scope of the invention. The present invention can be applied not only to the above-described isolation and enrichment of tritium from light water component, but also for separation and enrichment of the component of the heavy water.
CLAIM
1. A method for separation of hydrogen isotope, comprising the steps of: bringing a medium containing the hydrogen gas includes said hydrogen isotope, a water and water vapor in a reduced pressure condition of 90 kPa or lower, and controlling the process temperature in said medium in accordance with the pressure in said environment, regulation of the partial pressures of hydrogen gas and steam and separation efficiency control said hydrogen isotope from a gaseous hydrogen exchange reaction using a hydrogen-water system.
2. The method for allocating of claim 1, wherein the separation efficiency of said hydrogen isotope is optimized by controlling the pressure and the operating temperature of said medium.
3. The method for allocating of claim 1, wherein the hydrogen isotope is recovered as a liquid reacted water formed as a result of the first exchange reaction, in which water vapor intermediate containing said hydrogen isotope, is formed by the exchange reaction between hydrogen gas containing said hydrogen isotope and steam, and as a result of the second exchange reaction in which said liquid unreacted water containing an isotope of hydrogen, is formed by the exchange reaction between the intermediate water vapor and liquid water.
4. The method for allocating of claim 3, wherein the first exchange reaction is carried out using a catalyst located in the separation column.
5. The method for allocating of claim 3, wherein the second exchange reaction is carried out by feeding an intermediate steam countercurrent to the movement of liquid water in a separation column.
6. The method for allocating of claim 1, wherein said hydrogen isotope is tritium.
7. The method for allocating of claim 6, wherein said hydrogen gas is produced by electrolysis of water.
8. The method for allocating of claim 1, further comprising the step of detecting a leak of said medium in a separation process based on monitoring said fluid temperature increase in comparison with the process temperature.
9. A method for isolating an isotope of hydrogen, in which the medium containing the hydrogen gas comprises a hydrogen isotope, a water and water vapor, lead to conditions of reduced pressure and a certain temperature and said hydrogen isotope is separated from hydrogen gas, comprising the step of detecting a leak of the medium in the flow conditions separation process based on monitoring the temperature increase relative to the working medium temperature process.
10. An apparatus for separation of hydrogen isotope, comprising a separation column with a medium comprising hydrogen gas comprising said hydrogen isotope, a water and steam are designed to release the hydrogen isotope from the hydrogen gas by exchange reaction in the hydrogen-water system and temperature controllers providing medium temperature regulation in accordance with its pressure regulation of the partial pressures of hydrogen gas and water vapor and efficiency of regulation of the evolution of hydrogen isotopes of hydrogen gas.
11. The apparatus of claim 10, wherein the temperature controller so constructed that allow optimizing the efficiency of hydrogen isotope separation by pressure regulation and the operating temperature of said medium.
12. The apparatus of claim 10, wherein said temperature controllers record medium temperature rise as compared with an operating temperature associated with leakage of the medium from the separation column, and the evolution of hydrogen stopped from a gaseous hydrogen isotope exchange reaction by the hydrogen-water system, flowing in said separation column.
13. The apparatus of claim 10, wherein the separation column comprises a first metathesis catalyst to form an intermediate flowing steam containing hydrogen isotope exchange reaction as a result of hydrogen gas containing hydrogen isotope, and steam.
14. The apparatus of claim 13, wherein in said second separation column is carried out an exchange reaction between water vapor and intermediate liquid water to form a liquid reacted water containing hydrogen isotope.
15. The apparatus of claim 10, further comprising an electrolytic cell in which electrolytic water is obtained by hydrogen gas containing hydrogen isotope, which is tritium.
16. An apparatus for separation of hydrogen isotope, comprising a separation column with a medium comprising hydrogen gas comprising said hydrogen isotope, a water and steam are designed to release the hydrogen isotope from the hydrogen gas by exchange reaction in the hydrogen-water system, and temperature controllers intended for registration environment temperature increase in comparison with an operating temperature associated with leakage of the medium from the separation column, and stopping the operation of the hydrogen isotope separation of hydrogen gas flow resulting in a separation column exchange reaction into hydrogen-water system.
print version
Publication date 01.03.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.