| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2286210
![]()
BIFUNCTIONAL CATALYST AND A METHOD FOR PRODUCING A GASEOUS MIXTURE FROM DIMETHYL ETHER CONTAINED BY HYDROGEN BY HYDROGEN
The name of the inventor: Sukhe (RU); Volkova Galina Georgievna (RU); Belyaev Vladimir Dmitrievich (RU); Plyasova Lyudmila Mikhailovna (RU); Sobyanin Vladimir Alexandrovich
The name of the patent holder:
G.K. Boreskov Siberian Branch of the Russian Academy of Sciences
Address for correspondence: 630090, Novosibirsk, Acad. Lavrentyeva, 5, Institute of Catalysis named after. G.K. Boreskov, Patent Department, Т.D. Yudina
Date of commencement of the patent: 2005.10.17
The present invention relates to a catalytic method for performing a steam reforming reaction of dimethyl ether (DME) in order to obtain a hydrogen-rich gas mixture that can be used in hydrogen power, in particular as fuel for feeding fuel cells for various purposes. A bifunctional catalyst for the steam conversion of dimethyl ether containing acidic centers for the hydration of DME to methanol and copper-containing centers for the steam conversion of methanol, which is a copper-cerium oxide supported on alumina, is described. The method of obtaining hydrogen-enriched gas mixture by the interaction of DME and water vapor at a temperature of 200-400 ° C, a pressure of 1-100 atm, a molar ratio of H 2 O / DME equal to 2-10, is described also in the presence of the catalyst described above. The technical result is high hydrogen productivity, the production of a hydrogen-containing gas with a low carbon monoxide content at a water vapor / DME ratio equal to stoichiometric (H 2 O / DME = 3), which is of great technological importance.
DESCRIPTION OF THE INVENTION
The invention relates to a catalytic method for performing a steam reforming reaction of dimethyl ether (DME) in order to obtain a hydrogen-enriched gas mixture that can be used in hydrogen power, in particular as fuel for feeding fuel cells for various purposes, including fuel cells Installed on mobile vehicles.
At present, fuel cells are considered as an alternative and environmentally friendly source of electrical energy. Hydrogen or hydrogen-enriched gas mixture is the main fuel for feeding fuel cells and can be obtained by steam conversion of natural gas, gasoline, methanol and DME. Despite the developed infrastructure and the relatively low price of natural gas and gasoline, they are converted at high temperatures (above 600 ° C for natural gas and above 800 ° C for gasoline), and the resulting hydrogen-containing gas contains a large amount of carbon monoxide. Dimethyl ether, like methanol, can easily and selectively convert to hydrogen-containing gas at a relatively low temperature (150-300 ° C). At the same time, DME is corrosion-resistant and non-toxic in comparison with methanol.
It is known that dimethyl ether, like methanol, can be obtained by direct synthesis from synthesis gas (FSRamos, AMDuarte de Farias, LEPBorges, JLMonteiro, MAFraga, EFSousa-Aguiar, LGAppel, Role of dehydration catalyst properties on one-step DME synthesis over physical mixtures, Catalysis Today 101 (2005) 39-44, TN Fleisch, A. Basu, MJ Gradassi, JG Masin, Dimethyl ether: A fuel for the 21 st century. Studies Surface Science and Catalysis, vol . 107 (1997) p.117-125), direct synthesis of dimethyl ether may be more advantageous than the synthesis of methanol (T. Shikada, Y.Ohno, T.Ogawa, M.Ono, M.Mizuguchi, K.Tomura, K. Fujimoto, Direct Synthesis of Dimethyl Ether from Synthesis Gas, Studies Surface Science and Catalysis, vol. 119 (1998) p.515-520).
Given this, and the fact that the physical and chemical properties of DME are similar to those of liquefied petroleum gas (I.Dybjaer, JBHansen, Large Scale Production of Alternative Synthesis of Fuel from Natural Gas, Studies Surface Science and Catalysis, vol. 107 (1997) p. 99-118), the process of vapor conversion of DME in order to produce hydrogen for fuel cell supply is a serious alternative to the process of methanol vapor conversion.
It is known that the steam reforming reaction of DME can proceed sequentially in a two-stage scheme through the hydration of DME to methanol (1) and the vapor conversion of the methanol formed into the hydrogen-containing gas (2):
CH 3 OCH 3 + H 2 O = 2 CH 3 OH (1)
CH 3 OH + H 2 O = CO 2 + 3H 2 (2)
The total reaction:
CH 3 OCH 3 + 3H 3 O = 2 CO 2 + 6H 2 (3)
Two types of catalysts are known for the steam conversion of DME: a mechanical mixture of a DME hydration catalyst and a copper-containing methanol vapor conversion catalyst and a bifunctional catalyst containing surface acid sites for the hydration of DME and copper-containing centers for methanol vapor conversion.
The following systems are known, which are a mechanical mixture of a DME hydration catalyst and a copper-containing methanol vapor conversion catalyst. In work (Pat. RF 2,165,790, B 01 J 23/85, 27.4.2001), a mechanical mixture of supported heteropolyacids and copper-containing catalysts for the synthesis of methanol are used. In the work (Matsumoto T., Nishiguchi T., Kanai H., Utani K., Matsumura Y., Imamura S., Steam reforming of dimethyl ether over H-mordenite-Cu / CeO 2 catalysts, Applied Catalysis A: General 276 2004) 267-273) use a mechanical mixture of a copper-cerium catalyst and mordenite. The disadvantage of using a catalyst representing a mechanical mixture is its stratification into hydration catalysts of DME and methanol vapor conversion under the influence of vibration and, as a result, a decrease in the activity of the catalyst.
The closest to the claimed catalysts are bifunctional catalysts, which contain both types of centers on the surface: acid centers for the hydration of DME and copper-containing centers for methanol vapor conversion.
As bifunctional catalysts, Cu / Al2O3, Cu-Zn / Al2O3, Cu-Pd / Al2O3, Cu-Ru / Al2O3, Cu-Pt / Al2O3, Cu- Rh / Al 2 O 3 , Cu-Au / Al 2 O 3 , Cu / Ga 8 Al 2 O 15 (T. Matthew, Y. Yamada, A.Ueda, H.Shioyama, T. Kobayashi, Appl. : Gen. 286 (2005) 11, Takeishi K. "Suzuki H., Steam Reforming of Dimethyl Ether, Applied Catalysis A: General 260 (2004) 111; JP 2002263504 A2, September 17, 2002); Cu-Zn / Al2O 3 (JP 2003038957 A2, 12.02.2003) The reaction is carried out at a temperature of 350 ° C, a pressure of 1 atm. A disadvantage is the low hydrogen productivity, which does not exceed 65 mmol g -1 h -1 .
The invention solves the problem of developing a catalyst having high catalytic activity, selectivity and stability with respect to steam reforming of dimethyl ether (DME), as well as developing a high-performance process for the production of a hydrogen-enriched hydrogen mixture from DME using this catalyst.
The problem is solved by the development of a new catalytic system for obtaining hydrogen-enriched gas mixture by the interaction of dimethyl ether and water vapor, which is a bifunctional catalyst containing acidic centers for hydration of DME and copper-containing centers for methanol vapor conversion.
A catalyst is proposed for the preparation of a hydrogen-enriched gas mixture by the reaction of dimethyl ether and water vapor, which is a bifunctional catalyst containing on the surface hydration centers of dimethyl ether and methanol vapor conversion containing copper-cerium oxide supported on alumina.
The catalyst contains copper-cerium oxide in an amount of 1-20% by weight, the balance is alumina.
The weight ratio of Cu: Ce in the copper-cerium oxide is 1: 1-4: 1.
The problem is solved by the development of a method for obtaining a hydrogen-rich gas mixture by the reaction of dimethyl ether and water vapor in the presence of a bifunctional catalyst, which is a copper-cerium oxide supported on alumina.
The reaction is carried out at a temperature of 200-400 ° C, preferably 300-370 ° C, a pressure of 1-100 atm, preferably 1 atm, and a water / dimethyl ether H 2 O / DME ratio of 2-10, preferably 3.
A distinctive feature of the proposed bifunctional catalyst system is that copper-cerium systems supported on the support-alumina are used as the active component of methanol vapor conversion. In addition, alumina, used as a carrier, is a catalyst for the hydration of DME.
A distinctive feature of the proposed method for producing a hydrogen-rich gas mixture by the reaction of dimethyl ether and water vapor is the use of the above-described bifunctional catalyst.
Bifunctional catalysts are prepared by applying alumina in a solution of nitric acid salts of copper and cerium, taken in the required ratio, followed by drying and calcination in air at a temperature of 400-450 ° C.
The invention is illustrated by the following examples describing the composition of the catalysts and the results of their tests in the steam reforming reaction of dimethyl ether.
Example 1 .
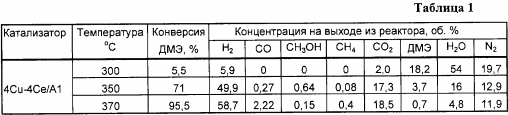
Steam conversion of dimethyl ether is carried out in a flow-through type installation in a quartz reactor with an internal diameter of 8 mm on a catalyst weighing unit of 0.4 g at a ratio of H 2 O: DME = 3: 1, contact time 10,000 h -1 , 350 ° C and 1 atm. The composition of the oxide catalyst in terms of metals is, by mass%: copper - 4, cerium - 4, the rest - aluminum. The results obtained are shown in Tables 1 and 6.
Example 1a . Analogously to Example 1, but the reaction was carried out at a temperature of 300 ° C., the results are shown in Table 1.
Example 1b . Analogously to Example 1, but the reaction was carried out at a temperature of 370 ° C., the results are shown in Table 1.
Example 2 .
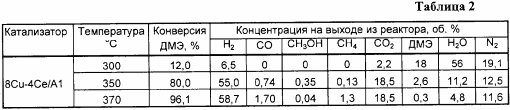
Analogously to Example 1, but the copper content is 8% by weight, the results are shown in Tables 2 and 6.
Example 2a . Analogously to Example 2, but the reaction was carried out at a temperature of 300 ° C., the results are shown in Table 2.
Example 2b . Analogously to Example 2, but the reaction was carried out at a temperature of 370 ° C, the results are shown in Table 2.
Example 3 .
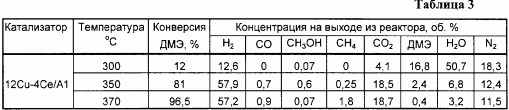
Similar to Example 1, but the copper content is 12% by weight, the results are shown in Tables 3 and 6.
Example 3a . Analogously to Example 3, but the reaction was carried out at a temperature of 300 ° C., the results are shown in Table 3.
Example 3b . Analogously to Example 3, but the reaction was carried out at a temperature of 370 ° C., the results are shown in Table 3.
Example 4 .
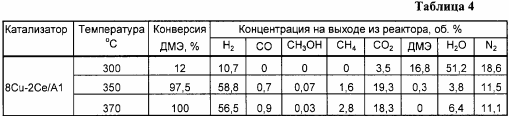
Similar to Example 2, but the cerium content is 2% by weight, the results are shown in Tables 4 and 6.
Example 4a . Analogously to Example 4, but the reaction was carried out at a temperature of 300 ° C., the results are shown in Table 4.
Example 4b . Analogously to Example 4, but the reaction was carried out at a temperature of 370 ° C., the results are shown in Table 4.
Example 5 .
Similar to Example 1, but the copper content is 6%, cerium is 6%. The results are shown in Tables 5 and 6.
Example 5a . Analogously to Example 5, but the reaction was carried out at a temperature of 300 ° C., the results are shown in Table 5.
Example 5b . Analogously to Example 5, but the reaction was carried out at a temperature of 370 ° C, the results are shown in Table 5.
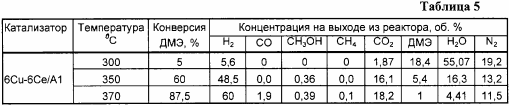
The above examples demonstrate the high activity, selectivity and stability of the proposed catalysts in the process of steam conversion of dimethyl ether into a gas mixture enriched in hydrogen.
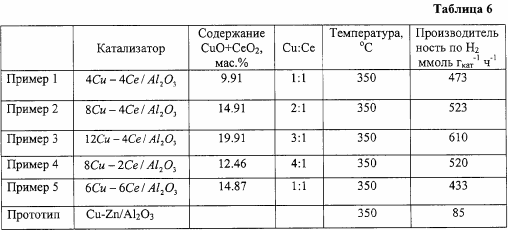
The use of the proposed catalysts makes it possible to increase hydrogen productivity by 8-10 times compared to the known one and to obtain a hydrogen-containing gas with a low carbon monoxide content at a water vapor / DME ratio equal to stoichiometric (H 2 O / DME = 3), which is of great technological importance .
CLAIM
A bifunctional catalyst for producing hydrogen-enriched gas mixture by the reaction of dimethyl ether and water vapor containing, on its surface, dimethyl ether hydration and methanol vapor conversion centers, and comprising copper oxide supported on alumina, characterized in that the catalyst contains copper Is a cerium oxide supported on alumina.
2. A catalyst according to claim 1, characterized in that the content of the copper-cerium oxide is 1-20% by weight, the balance is alumina.
3. The catalyst of any one of claims 1 and 2, wherein the weight ratio of copper to cerium in the copper-cerium oxide is 1: 1-4: 1.
4. A process for producing a hydrogen-rich gas mixture by reacting dimethyl ether and water vapor in the presence of a bifunctional catalyst containing on the surface hydration centers of DME and methanol vapor conversion and comprising copper oxide supported on alumina, characterized in that as a catalyst A copper-cerium oxide supported on alumina is used.
5. A process according to claim 4, characterized in that the reaction is carried out at a temperature of 200-400 ° C, preferably 300-370 ° C, a pressure of 1-100 atm, preferably 1 atm, and a water / dimethyl ether mole ratio of 2- 10, preferably 3.
print version
Date of publication 01.03.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.