| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2165790
![]()
CATALYST AND METHOD FOR PRODUCING enriched in hydrogen gas mixture
From dimethyl ether
Name of the inventor: Belyaev VD .; Volkov GG .; Galvita VV .; Demeshkina MP .; Itenberg I.SH .; Minyukova, etc .; Semin GL .; Sobyanin VA .; St. George TM
The name of the patentee: Institute of Catalysis. GK Boreskov SB RAS
Address for correspondence: 630090, Novosibirsk, Lavrentiev 5, Institute of Catalysis.. GK Boreskov, patent department, Yudina TD
Starting date of the patent: 2000.03.13
The invention relates to a steam reforming reaction of dimethyl ether to obtain enriched in hydrogen gas mixture which can be used in hydrogen energy, particularly as fuel for the fuel cell for various purposes, including for fuel cells mounted on mobile facilities. The task to be solved by the present invention is to provide a novel catalyst system having high catalytic activity, selectivity and stability to steam reforming of dimethyl ether (DME), but also the development process of obtaining from DME gaseous mixture enriched in hydrogen, using this catalyst system. The problem solved by the development of a catalyst which is a mechanical mixture of ether hydration catalyst and a copper-containing catalyst for steam conversion of methanol, and the ether hydration catalyst is a Si- or F, or W-MO heteropolyacid or Na-, Mg-, Cu- and Zn- salt deposited on SiO 2 or Al 2 O 3 in an amount of 1-50 wt.%, the balance carrier SiO 2 or Al 2 O 3, and a process for producing hydrogen-rich gas mixture by reacting dimethyl ether and water vapor in the presence of said catalyst. The catalysts have a wide possibility of variation of chemical composition. Using them allows you to reduce the operating temperature of the process and to implement it at a ratio of steam / DME, equal to the stoichiometric (H 2 O / DME = 3), which is of great technological importance.
DESCRIPTION OF THE INVENTION
The invention relates to a catalytic method of steam reforming of dimethyl ether reaction to obtain enriched in hydrogen gas mixture which can be used in hydrogen energy, particularly as fuel for the fuel cell for various purposes, including for fuel cells mounted on a movable means. Fuel cells are currently being considered as a viable alternative energy sources known in the mobile means, such as internal combustion engines and batteries. In this case, the fuel for fuel cells is hydrogen or of hydrogen-rich gas mixture.
There are two main ways of supplying hydrogen to the fuel cell (JV Ogden, MM Steinbugler, TG Kreutz, A Comparison of Hydrogen, Methanol and Gasoline as Fuels for Fuel Cell Vehicles: Implications for Vehicle Design and Infrastructure Development, Journal of Power Sources, vol 79. (1999), p. 143-168). In the first method, hydrogen is fed in pure form from a storage vessel where it is in a compressed state. The disadvantage of this method is the need for equipment operating at high pressure, which complicates the process and increases the cost of increased consumption of materials and systems. According to the second scheme, the hydrogen obtained in the catalytic chemical process of hydrogen carriers, namely hydrocarbons or methanol directly on a mobile vehicle.
It is known that the process of catalytic steam reforming of methanol is considered as the main method for producing hydrogen-rich gas mixture directly onto a mobile vehicle fuel cell power order. This process is carried out in the presence of catalysts containing, for example, palladium (Iwasa N .; Kudo S .; Takahashi H .; Masuda S .; Takezawa N., Hight Selective Supported Pd Catalysts for Steam Reforming of Methane, Catalysis Letters, vol. 19, (1993) N 2-3, p 211-216), or copper and zinc (Wang D .; Ma L;. Jiang CJ; Trimm DL; Wainwright MS; Kirn DH, The Effect of zinc Oxide in Raney copper Catalysts on Methanol Synthesis , Water Gas Shift and Methanol Steam Reforming Reaction, Studies In Surface Science And Catalysis, vol 101 (1996), p 1379-1387; Idem RO, Bakhshi NN, Production of Hydrogen from Methanol over Promoted Coorecipitated Cu-Al Catalysts:.. The Effects of Varies Promotors and Catalyst Activation Methods, Ind. Eng. Chem. Res., vol. 34 (1995), p. 1548-1557).
It is known that dimethyl ether, as well as methanol, may be prepared by direct synthesis from synthesis gas (Rouchi AM, Dimethyl Ether es Alternative Diesel Fuel C & EN, May 25 (1995), p 37-39;.. Fleisch T. N., Basu A., Gradacci MJ, Masin JG, dimethyl ether:.. A fuel for the 21 st century, Studies in Surface Science and Catalysis, vol 107 (1997), p 117-125), and dimethyl ether synthesis economically more favorable than the methanol synthesis (Shikada T., Ohno Y., Ogava T. Ono M, Mizuguchi Tomura Fujimoto K. Direct synthesis of Dimethyl Ether from synthesis Gas, Studies in Surface Science and Catalysis, vol. 119 (1998) p. 515-520).
Given this, and the fact that the physical and chemical properties similar to those of DME liquefied petroleum gas (Dybkjaer I., Hansen JB, Large Scale Production of Alternative Synthetic Fuel from Natural Gas, Studies in Surface Science and Catalysis, vol. 107 (1997) , p 99 -. 118), the process of steam reforming DME to produce hydrogen on a mobile vehicle to power a fuel cell is a serious alternative to the process of steam reforming of methanol.
Known two-stage process of producing DME to hydrogen rich gas mixture (US Pat. N 5,626,794, C 07 C 01/00, 1997). In the first step the catalytic steam reforming of dimethyl ether over catalysts containing copper and zinc in elemental form and not comprising alkali metals (first step) to form hydrogen and carbon monoxide. In the second stage, the catalytic steam reforming of carbon monoxide on catalysts comprising the oxides of copper, zinc, chromium or iron. The resulting two-stage process, the gas mixture used for combustion to produce heat and driving the gas turbine to produce mechanical or electrical energy. The disadvantage of this method is receiving the first stage diluted gas mixture through the use of an inert diluent gas (nitrogen), a high first stage flow temperature (above 350 o C) and a low degree of conversion of dimethyl ether, which is at temperatures below 350 o C is not more than 88% .
Is the most similar one-step process for producing hydrogen-rich gas mixture by reacting dimethyl ether and steam by reacting CH 3 OCH 3 + 3H 2 O = 2CO 2 + 6H 2 in the presence of a mechanical mixture of two catalysts:
(1) ether hydration catalyst constituting aluminosilicate ZSM in the hydrogen form or SIRAL 5, and (2) methanol decomposition catalyst (Pat. US N 5837217, C 01 B 03/02, 17.11.98).
The disadvantage of this method and the catalyst is that complete conversion of the dimethyl ether is achieved at a sufficiently high temperature ![]() 300 o C and at an elevated ratio H 2 O / DME = 4: 1 compared to the stoichiometric H 2 O / DME = 3. This reduces the overall efficiency of steam reforming of dimethyl ether in the process of hydrogen-containing gas.
300 o C and at an elevated ratio H 2 O / DME = 4: 1 compared to the stoichiometric H 2 O / DME = 3. This reduces the overall efficiency of steam reforming of dimethyl ether in the process of hydrogen-containing gas.
The task to be solved by the present invention is to provide a novel catalyst system having high catalytic activity, selectivity and stability to steam reforming of DME, but also the development process of obtaining from dimethyl ether gaseous mixture enriched in hydrogen using this catalyst system, which will reduce the operating temperature and water vapor content at the reactor outlet, thereby increasing process efficiency.
The problem is solved for the development of a catalyst for hydrogen-rich gas mixture by reacting dimethyl ether and water vapor, which is a mechanical mixture of ether hydration catalyst and a copper-containing catalyst for steam conversion of methanol, and the ether hydration catalyst is a Si- or P-, Mo- or W - heteropolyacid or Na-, Mg-, Cu- or Zn-salt deposited on SiO 2 or Al 2 O 3 in an amount of 1-50 wt%, the rest -. carrier SiO 2 or Al 2 O 3.
The problem is solved by providing a method for producing hydrogen-rich gas mixture by reacting dimethyl ether and steam in the presence of ether hydration catalyst and a copper-containing catalyst for steam conversion of methanol, as with ether hydration catalyst used Si- or P-, Mo- or W-heteropoly or Na, Mg-, Cu- or Zn-salt supported on a carrier. Ether hydration catalysts and steam reforming of methanol are used in a weight ratio of from 1: 5 to 5: 1. The reaction is carried out at 150-450 o C, 1-100 atm and a molar ratio of water / dimethyl ether H 2 O / DME 2-10.
The process proceeds according to the reactions:
CH 3 OCH 3 + H 2 O = 2CH 3 OH; (1)
CH 3 OH + H 2 O = 3H 2 + CO 2 (2)
CO + H 2 = CO 2 + H 2 O (3)
the overall reaction:
CH 3 OCH 3 + 3H 2 O = 2CO 2 + 6H 2. (4)
The hallmark of the proposed catalytic system, which is a mechanical mixture of two catalysts is that as used DME hydration catalyst a heteropolyacid (HPA) or their salts supported on a carrier; as the steam reforming of methanol catalyst - known copper-containing catalysts, such as Cu-Zn-Al - the methanol synthesis catalyst (Pat RF N 2055639, B 01 J 37/08, Bulletin N 7 18.06.93..), Cu-Zn-AI ( Cr) and Cu-Mg-vapor conversion of CO catalysts (US Pat. RF N 2118910, B 01 J 37/08, Bull. 26 N, 03.26.97), (Ed. St. USSR 223069, Bul. N 33, 1978) .
The composition and methods for producing copper-containing catalysts are shown in the above patents.
ether hydration catalysts have the following composition:
H 4 [Si (P)] [Mo (W)] 12 O 40 or Na-, Mg-, Cu-, Zn-salts on carriers such as SiO 2 and Al 2 O 3.
The hallmark of the process for producing hydrogen-rich gas mixture by reacting dimethyl ether and water vapor is to use the hydration step to a novel catalyst based on ester heteropolyacids and salts thereof as proposed above.
The invention is illustrated by the following Examples which describe methods for preparing the catalysts and the results of tests in steam reforming of dimethyl ether reactions.
Steam reforming of dimethyl ether is carried out in a flow-in glass or quartz reactor diameter 8 mm rigging mechanical mixture of the two catalysts at a ratio of 3 g water / DME = 3: 1-5: 1, the contact time of 1200-5000 h -1 200- 350 o C and 1-5 atm. The rigging weight ratio of the catalyst to the copper-containing catalyst based on HPA or their salts can vary in the range of 1 / 5-5 / 1.
Ether hydration catalyst is prepared by impregnating the carrier with an aqueous solution of their salts or HPA wetness followed by heat treatment in air at 250-300 o C.
Example 1. Catalyst - silicotungstic GIC / Al 2 O 3 is prepared:
a) 44.6 g of Si-W-CPC dissolved in water while heating so that the solution volume was 60 ml;
b) 100 grams (2.1 mm) ![]() -Al 2 O 3 with a surface area of 200 m 2 / g and a pore volume of 0.6 cm 3 / g were impregnated with the resultant solution under stirring, then dried at 25 o C for 20 hours at 100 o C for 4 hours and calcined at air at 300 o C for 4 hours.
-Al 2 O 3 with a surface area of 200 m 2 / g and a pore volume of 0.6 cm 3 / g were impregnated with the resultant solution under stirring, then dried at 25 o C for 20 hours at 100 o C for 4 hours and calcined at air at 300 o C for 4 hours.
Example 2. Catalyst - silicotungstic HPA / SiO 2 prepared:
a) 15 g of Si-W-CPC dissolved in water while heating so that the solution volume was 50 ml;
b) 10 g of SiO 2 powder (0.5-1.0 mm) is impregnated with stirring 15 ml of the resulting solution, followed by drying at 25 o C for 20 hours at 100 o C for 4 hours and calcined in air at 300 o C for 4 hours.
Example 3. Catalyst - phosphomolybdic GIC / Al 2 O 3 is prepared:
a) 20 g of P-Mo HPA dissolved by heating in water so that the solution volume was 65 ml;
b) 10 grams (2.1 mm) ![]() -Al 2 O 3 is impregnated with stirring 6 ml of the resulting solution was further dried at 25 o C for 20 hours at 100 o C for 4 hours and calcined in air at 250 o C for 4 hours. Then the catalyst was impregnated and calcined again. This treatment was repeated 3 times.
-Al 2 O 3 is impregnated with stirring 6 ml of the resulting solution was further dried at 25 o C for 20 hours at 100 o C for 4 hours and calcined in air at 250 o C for 4 hours. Then the catalyst was impregnated and calcined again. This treatment was repeated 3 times.
Example 4. Catalyst - magnesium salt of silicotungstic HPA / SiO 2 prepared:
a) 25 g of Si-W-CPC dissolved in water while heating so that the volume of the solution was 40 ml, then to this solution was added 0.56 g of MgO, and the mixture was heated until complete dissolution of the oxide;
b) 10 g of powder (0.5-1.0 mm) SiO 2 (XK a pore volume of 1.5 cm 3 / g) was impregnated with stirring 15 ml of the resulting solution, followed by drying at 25 o C for 20 hours at 100 o C for 4 hours and calcined in air at 300 o C for 4 hours.
Example 5. Steam reforming of dimethyl ether in the enriched in hydrogen mixture is carried out in a flow reactor with a mechanical mixture consisting of a Cu-Mg-oxide catalyst for steam conversion CO and catalyst hydration ester prepared by the method described in Example 1, taken with a weight ratio of 3 / 4 respectively. The results are shown in Table 1.
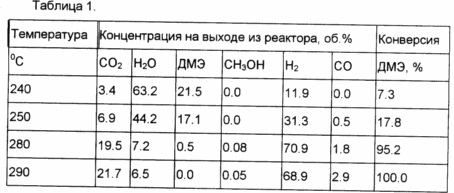
Example 6. In a process analogous to that in Example 5, DME is converted to enriched in hydrogen mixture with a mechanical mixture of Cu-Zn-Al-methanol synthesis catalyst and the catalyst prepared by the method described in Example 2. The weight ratio of the catalyst mixture 1.1. The results are shown in Table 2.
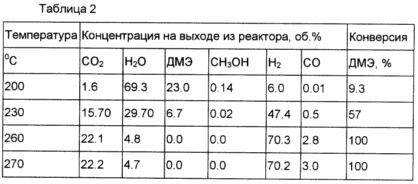
Example 7 In a process analogous to that in Example 5 was converted to DME to hydrogen rich mixture to a mechanical mixture of Cu-Zn-Al-CO conversion of the catalyst and the catalyst prepared by the method described in Example 3. The weight ratio of the catalyst mixture 1.1. The results are shown in Table 3.
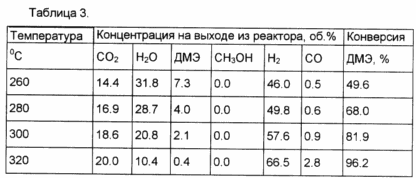
Example 8. In a process analogous to that in Example 5 was converted to DME to hydrogen rich mixture to a mechanical mixture of the Cu-Mg-oxide catalyst and CO conversion of the catalyst prepared by the method described in Example 4. The weight ratio of catalysts 1 mixture /1. The results are shown in Table 4.
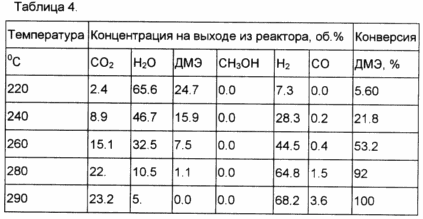
These examples demonstrate methods for preparing catalysts, and a high activity, selectivity and stability of the proposed catalysts.
The catalysts have a wide possibility of variation of chemical composition. Using the proposed catalysts can lower the temperature of the process and to implement it at a ratio of steam / dimethyl ether equal to the stoichiometric (H 2 O / DME = 3), which is of great technological importance.
CLAIM
1. A catalyst for producing hydrogen-rich gas mixture by reacting dimethyl ether and water vapor, which is a mechanical mixture of catalyst and a copper-containing ether hydration catalyst steam reforming of methanol, characterized in that the ether hydration catalyst is a Si- or P- Mo- and W- heteropolyacid or Na-, Mg-, Cu- or Zn-salt deposited on SiO 2 or Al 2 O 3.
2. A catalyst according to claim 1, characterized in that in the ether hydration catalyst contains heteropoly acid or salt thereof in an amount of 1 - 50 wt%, the rest -. Carrier SiO 2 or Al 2 O 3.
3. A method for producing hydrogen-rich gas mixture by reacting dimethyl ether and steam in the presence of ether hydration catalyst copper-containing catalyst and steam reforming of methanol, characterized in that the ether hydration catalyst is used Si- or P- Mo- or W-heteropolyacid or Na-, Mg-, Cu- or Zn-salt supported on a carrier.
4. The method of claim 3, wherein the ether hydration catalyst and steam reforming of methanol are used in a weight ratio of 1: 5 - 5: 1.
5. The method according to claim 4, characterized in that the reaction is carried out at 150 - 450 o C, 1 - 100 atm and the molar ratio of water / dimethyl ether H 2 O / DME 2 - 10.
print version
Publication date 01.03.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.