| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2091294
![]()
A METHOD FOR OBTAINING A PERO-HYDROGEN, A CATALYST FOR THE IMPLEMENTATION
AND A METHOD OF OBTAINING A CATALYST
The name of the inventor: Dubiaga NA; Bondartzova II; Morozov VS; Pupovsky AF; Levchenko AL; Rudoy Yu.S .; Velichko AS; Morozov EV; Podorozhnyak A.Ya.
The name of the patent holder: The State Research and Design Institute of the Nitric Industry and Organic Synthesis Products
Address for correspondence:
Date of commencement of the patent: 1991.01.29
The invention relates to a process for the production of parahydrogen by low-temperature conversion of orthohydrogen, to the composition of the catalyst for its preparation, and to a method for producing this catalyst. The object of the invention is, accordingly, to increase the productivity of a unit of the reaction volume, to increase the activity and strength of the catalyst, and to shorten the formation time of the catalyst precipitate. Hydrogen containing orthohydrogen is contacted under cryogenic conditions at a space velocity of 4-4.5 × 10 4 h -1 , with the hydrated iron (III) catalyst molded. The catalyst is then promoted by hydrated oxides of the general formula FeMe (OH) 3 or FeMe (OH), where Me is molybdenum, tungsten, chromium, vanadium and cobalt contained in the mixture at certain concentrations. The catalyst is prepared by adding equnormal aqueous sodium hydroxide solution at a temperature of 288-303 K to a 2-6 H iron (III) chloride solution, the resulting iron (III) oxide sol is then mixed with the slurry of the anodic dissolution process of the steel at a ratio of 1  0.5-1), based on iron; The resulting precipitate is formed, the precipitate is washed from sodium and chlorine ions by decanting, dried, molded and activated in a hydrogen stream at T = 373-423 K.
0.5-1), based on iron; The resulting precipitate is formed, the precipitate is washed from sodium and chlorine ions by decanting, dried, molded and activated in a hydrogen stream at T = 373-423 K.
DESCRIPTION OF THE INVENTION
The invention relates to the production of parahydrogen by low-temperature conversion of orthohydrogen, to a catalyst for its implementation and to a method for producing said catalyst. The inventions of this group are intended for use in the production of liquid hydrogen.
The prospect of a rapid growth in the production and storage of liquid hydrogen necessitates the first-order resolution of issues related to the organization of modern production of parahydrogen by low-temperature conversion, the development of modern catalyst formulations for this process and methods for their preparation.
Under normal conditions, at temperatures above 273 K, hydrogen is a mixture of isomers: 25% steam and 75% orthohydrogen. The existence of hydrogen simultaneously in two isomeric forms causes certain difficulties in its liquefaction, since the isomers of hydrogen, completely unchanged in their chemical properties, have pronounced differences in physical properties. Moreover, the specific heat of hydrogen liquefaction at 20 K is 0.216-0.228 kcal / mol, while the heat of the ortho-para conversion at the same temperature is 0.337 kcal / mol, i.e. Is 4.5 times higher.
At the same time, when the temperature decreases, the thermodynamically equilibrium isomer distribution changes: at a temperature of 78 K (boiling point of liquid nitrogen), the equilibrium mixture contains 50.4% of parahydrogen, at a hydrogen liquefaction temperature at atmospheric pressure of 20.4 K and lower, the equilibrium mixture Consists essentially of only a more ordered isomer-para-hydrogen.
Therefore, simple hydrogen liquefaction, similar to other gases, for example, neon or helium, results in the "normal" hydrogen being liquefied, then the spontaneous process of conversion ortho-pair begins, which develops with acceleration in time. Liquid hydrogen vigorously boils and evaporates, which can lead to serious accidents. This circumstance explains the need to implement the ortho-para conversion in the production of liquid parahydrogen, the storage of which does not cause difficulties [1]
Growth of production, consumption and storage of liquid hydrogen required the development of modern large-scale (and mobile) installations; The latter impose certain regulations on the process of obtaining para-hydrogen, the catalysts used and the methods for their production [1]
Such regulations are:
1. Catalyst activity. Usually, the tests are carried out under ortho-para conversion conditions of the so-called "normal" hydrogen, i.e. Containing 25% parahydrogen and 75% orthohydrogen at the boiling point of liquid nitrogen (77 K). In accordance with the requirements for the catalyst, the activity should not be lower than 3 · 10 -3 mol / g; More active catalysts are preferred.
The activity of the catalyst is also a parameter that determines the productivity of a unit of the reaction volume, which determines the dimensions and material consumption of the installation.
2. Catalyst activation conditions. The final activation of the catalyst must be carried out directly in the reactor of the ortho-para conversion, since it is difficult to avoid entering the system of oxygen, water vapor, etc. during the reactor loading. Safety requirements impose technological limitations: the temperature in the reactor can not be higher than 423 K, and the pressure in the reactor during the activation process is below 10 4 Pa.
The known methods for producing parahydrogen by low-temperature ortho-para conversion, involving contacting a mixture of ortho-para-isomers under cryogenic conditions with a pre-activated shaped catalyst, do not satisfy the above-mentioned regulations.
At the same time, either insufficiently active catalysts are used, which entails low specific process productivity (low parahydrogen yield per unit of reaction volume) or involve the use of catalysts that need to be activated in the reactor under unacceptable activation conditions (vacuum, 423 K).
There are also known methods for producing para-hydrogen using very active catalysts such as "Metal on a carrier", but they also require the activation of catalysts in the reactor under unacceptably stringent conditions (temperature over 443 K, vacuum), which makes these processes inessential Action and short-term industrial equipment [2]
A special position among a number of analogs is the method of obtaining a catalyst for the production of parahydrogen based on metallic ruthenium on a carrier, which provides a high capacity of a unit of the reaction volume to 24.7 mmol / cm 3 · s. However, due to the lack of industrial extraction of ruthenium now and in the near future, the invention does not have any visible prospects for industrial use.
A number of hydroxides used as a catalyst in the production of parahydrogen were studied. At a temperature of 78 K, the following results were obtained: the catalyst-NiO · Cr 2 O 3 mixture provides a specific productivity of 1.5-1.7 mmol / cm 3 · s; The following catalysts - specific productivity, mmol / cm 3 · s:
Cr (OH) 3 0.56-0.73
Mo (OH) 2 0.73-1.2
Fe (OH) 3 1.0-2.3
Ni (OH) 2 0.44-0.68
Co (OH) 3 0.24-0.28
From the data given, it can be seen that the greatest productivity per unit volume of the reaction equipment is provided by catalysts based on iron hydroxides. In addition to hydroxide Fe (OH) 3, high activity is provided by fine crystalline goethites of lamellar system ![]() -Fe (OH). Anhydrous a-Fe 2 O 3 oxide is much less active [3] This method of producing para-hydrogen and the composition of the catalyst are the closest to the proposed.
-Fe (OH). Anhydrous a-Fe 2 O 3 oxide is much less active [3] This method of producing para-hydrogen and the composition of the catalyst are the closest to the proposed.
The hydrated ferric oxide (III) formed in practice can be expressed by the chemical formula FeO (OH). In general, the hydrated oxides of the transition metals (III) of the hexagonal polymorph can be expressed by the general formulas: MeOOH or Me (OH) 3 .
The object of the invention is to increase the productivity of a unit of reaction volume.
To achieve the above object, the present invention provides a process for the production of parahydrogen by low-temperature catalytic conversion of orthohydrogen, comprising contacting hydrogen-containing hydrogen fluoride in cryogenic conditions with a hydrated iron (III) oxide shaped catalyst of a hexagonal polymorph, wherein, according to the invention, A catalyst promoted by the hydrated oxides of molybdenum, tungsten, chromium, vanadyl and cobalt of the general formula FeMeO · OH or FeMe (OH) 3 is used and the process is carried out at a hydrogen space velocity of 4.0-4.5 × 10 4 h -1 .
The term "cryogenic conditions" in this description is of general use and covers temperatures up to 100 K.
A second invention of the claimed group is a catalyst for carrying out said process.
Regulation 1 and 2, relating to a process for the production of para-hydrogen by low-temperature catalytic conversion of orthohydrogen, fully apply to the second invention of the claimed group.
Most of the known catalysts for conducting ortho-vapor conversion of hydrogen can be divided into two main types.
First, they are catalysts containing very fine crystallites of the reduced metal on a porous carrier, most often oxide. Such systems include catalysts containing metallic ruthenium on a carrier of active carbon, alumina, silica gel and the like. [4] metallic nickel on silica gel [2]
The specific activity of such catalytic systems is very high, for example, for a catalyst of 25% Ru / C it reaches 22.1-24.7 mmol / g. The activity of the catalyst "nickel on silica gel" reaches 3.77 mmol / g. A common disadvantage, excluding the possibility of using these catalysts, is the need to restore the metal compound to the metallic state. The recovery of the metal-carrier system of pyrophoric, which makes it impossible to restore them outside the conversion reactor. By virtue of regulation 2, it is also impossible to carry out the reduction in the conversion reactor itself, since such activation requires high temperatures 623-873 K.
In addition, ruthenium has no visible prospects for industrial production now and for the foreseeable future.
The second group of catalysts includes systems based on oxides and hydroxides of trivalent metals. These catalysts find industrial application in the production of parahydrogen [1]. As noted above, the most active catalysts are based on iron hydroxides and hydrated iron oxides.
The most active catalyst for this group is the hydrated iron (III) oxide of the hexagonal polymorph (a compound of the general formula a-FeO (OH) [3]).
Promising and relevant is the development of more active catalysts, which have improved physical and mechanical properties.
The object of the invention is to increase the activity and strength of the catalyst.
To achieve this object, a catalyst is provided for carrying out a process for the production of parahydrogen by a low-temperature conversion of orthohydrogen based on a hydrated iron (III) oxide molded to a hexagonal polymorph, wherein, in accordance with the invention, the catalyst further comprises promoters, hydrated oxides of molybdenum, tungsten, chromium, vanadyl and Cobalt of the general formula FeMeO (OH) or FeMe (OH) 3 at the ratio of the ingredients, atomic proportions: Fe (0.92-0.96), Mo (0.0075-0.019), W (0.00048-0.0011) , Cr (0.011-0.025), V (0.005-0.012), Co (0.012-0.029).
The proposed catalyst has an activity of 4.5-4.6 mmol / g. And strength of 342-365 kg / cm 2 .
The ability of various trivalent metal cations to the isomorphic substitution of iron oxide is known and used in the prescribing of ethylbenzene dehydrogenation catalysts [5], however, no information has been found in the literature that the introduction of these promoters can lead to an increase in catalytic activity and catalyst strength for the low-temperature ortho-para process Conversion of hydrogen. This result does not in any way follow from the known properties of cation-promoters studied in [1], all of them are inferior to hydrated iron (II) oxide.
A third aspect of the invention of the claimed group is a method for preparing a catalyst for carrying out a process for producing para-hydrogen by low-temperature conversion of orthohydrogen.
Suitable methods for the industrial application of catalysts for the same purpose should be activated at a temperature of no higher than 423 K and a pressure of not less than 10 4 Pa. A method for the preparation of a catalyst is known in which an equimodium aqueous sodium hydroxide solution is added at a temperature of 208-303 K to a 2-6 H aqueous solution of iron (III) chloride to obtain an iron (III) hydroxide sol, then a precipitate of iron (III) hydroxide is formed, By settling, the precipitate is washed from sodium and chlorine ions by decanting, dried, molded and activated by the catalyst in a stream of hydrogen at 373-423 K.
This method produces a catalyst satisfying the above requirements. However, it has a long duration of the processes of formation and washing of the sediment from sodium and chlorine ions.
These operations cyclically repeat 3-5 times. Due to the fact that the precipitate prepared for the first decantation is formed for a long time 6-8 hours, it is this operation that determines to a certain extent the duration of the whole process of catalyst preparation (up to 12 hours).
The aim of the claimed method is to shorten the time of precipitate formation.
To achieve this object, according to the invention, according to the invention, there is provided a method of preparing a catalyst in which an equimodium aqueous sodium hydroxide solution is added to a 2-6 H aqueous solution of ferric chloride at a temperature of 288-303 K to produce an iron (III) hydroxide sol. This sol is mixed with the slurry obtained by anodic dissolution of the steel at a ratio of 1  0.5-1) (based on iron).
0.5-1) (based on iron).
Anodic dissolution of P6M5K5 steel is carried out on an industrial scale to form products of complex configuration. The process is carried out in a solution of sodium and / or potassium nitrates with a concentration of 7-10 wt. Typical dissolution conditions include: an electrolysis pressure of 5-6 kg / cm 2 , a total current of 2000-2500 A, a voltage of 12 V, a dissolution temperature below 50 ° C, a cathode made of EIT steel.
The production waste is a 75-85% moisture sludge containing products of anodic dissolution of P6M5K5 steel. The slurry contains the components preferably at the following ratio, wt. Molybdenum 4.8-5.3, tungsten 5.7-6.7, chromium 3.8-4.3, vanadium 1.7-2.1, cobalt 4.5-5.5, carbon 0.8- 0,9, iron up to 100.
The humidity of the slurry is 75-85%
During the preparation, this slurry is mixed with the iron hydroxide sol in the atomic ratio of (0.5-1): 1 (based on iron). The sludge itself is volumetric, not filterable, it is practically impossible to wash it off from ions and to prepare a catalyst.
Example 1 . In a 3 liter flask equipped with a turbine stirrer, 0.9 L of water (freshly boiled bidistillate) is charged and 269 g (3 equivalents) of iron (III) hexahydrate FeCl 3 · 6H 2 O crystals are dissolved therein with stirring. The resulting solution of iron (III) chloride with 3H concentration is cooled to 289 ± 1 K and with a stirred stirrer, a 3H sodium hydroxide (CHDA) solution in freshly boiled bidistallate is added dropwise. The rate of addition of sodium hydroxide is chosen so that the temperature in the flask does not rise above 303 K. After reaching a pH of 7.5 ± 0.1, which is controlled by a potentiometer, the supply of sodium hydroxide is stopped, the iron (III) hydroxide sol is formed. Turn off the stirrer. The precipitate is formed after 3 hours. After this, the mother liquor is decanted and poured into a flask of 2 liters of freshly boiled bidistillate. The washing is repeated six times. In the washing water, the content of sodium and chlorine ions is monitored. After six washes (total washing time 8.1 hours), the total content of sodium and chlorine atoms is about 1 g / l. The precipitate is transferred to a filter, dehydrated, dried in air at (50 ° C.) 323 K to an air-dry state, ground, a 20-32 mesh fraction is taken. (0.495-0.833 mm). A sample of a selected fraction of 10 cm 3 (12 g) is loaded into the reactor with a 9 mm diameter tube of molybdenum glass. The catalyst bed is limited by a glass wool nozzle on both sides. The tube is thermostated at 418 ± 5 K and at this temperature is activated with hydrogen for 5 hours. The hydrogen flow rate is 5 l / h. Hydrogen is purified from traces of oxygen by passing through an aluminum-platinum catalyst AP-65 and drained in a cryogenic trap cooled with liquid nitrogen. Activation is carried out until the hydrogen composition is supplied to the tube and withdrawn from the tube + 1 h. The tube is then immersed in a liquid nitrogen tank of 77-78 K and hydrogen is purified and dried in the manner indicated above. The hydrogen feed rate is adjusted so that the parahydrogen content in the effluent stream is 37.5-37.9%, which corresponds to a 50% conversion of the "normal" hydrogen. The specific catalytic activity is then calculated, which is 3.9 mmol / g. And a reaction volume capacity of 4.68 mmol / g. from. The composition of the catalyst is expressed by the general formula a FeO (OH).
Example 2 . Example 1 is repeated, but after the iron hydroxide sol is formed, 650 g of slurry from an anodic dissolution bath of P6M5K5 steel with a moisture content of 82% (atomic ratio of iron in the ash and in the precipitate of 1: 2) is added to it, then the mixer is turned off and 1 l of the mixture is left in the flask .
Formation of the sediment is completed after 12 minutes.
Washing is then carried out as in Example 1, but the total residual sodium and chlorine content of 1 g / l in the sludge is reached after only 4 washes. The total time for washing is 3 hours.
The specific catalytic activity is 4.5 mmol / g. The unit volume of the reaction volume is 5.4 mmol / cm 3 s.
The composition of the catalyst is expressed by the general formula, atomic fractions: Fe 0.9200, Mo 0.0130, W 0.0011, Cr 0.0250, VO 0.0120, Co 0.0289, FeO (OH).
The precipitate is isomorphic to a-FeO (OH).
Example 3 . Example 2 is repeated, but hydrogen activation is carried out at a temperature of 388 ± 5 K for 7 hours.
A catalyst sample with a specific catalytic activity of 4.5 mmol / g is obtained. The density of the sample is 1.1 g / cm 3 , specific unit capacity of the reaction volume is 4.95 mmol / cm 3 .
The composition of the catalyst is expressed by the general formula, atomic fractions: Fe 0.9200, Mo 0.0130, W 0.0011, Cr 0.02500, VO 0.0120, Co 0.0289, Me (OH) 3 .
Examples 4-6 .
Using the procedure of Example 2, samples of catalysts 4, 5, 6 are prepared. At the same time, precipitates are taken from various electrolysis baths, the amount of precipitation and the activation temperature in the hydrogen current are changed.
Compositions of catalysts, the duration of the process of formation and washing of the precipitate, data on the specific catalytic activity and specific unit capacity of the reaction volume of all catalyst samples are given in the table.
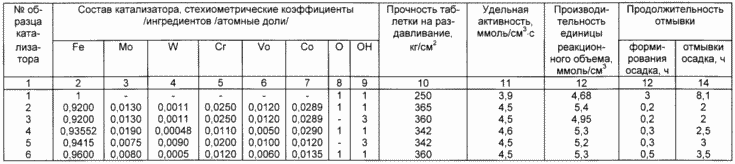
Columns 2-9 show the catalyst composition, in 1 reference standard, in column 10, the mechanical strength of the standard catalyst pellet used for the so-called "bath" low-temperature ortho-para conversion process. For the preparation of a standard tablet fraction of ~ 32 mesh. Obtained after crushing the dried cake, is pressed with a force of 5000 kg / cm 2 into a 5 × 5 mm tablet and subjected to a crushing test at the end.
It can be seen from the table that the method according to the claimed invention exceeds in all respects the known method, the catalyst according to the claimed invention exceeds the known catalyst, and the method of preparation of the catalyst in accordance with the claimed invention allows to reduce the formation and washing time of the precipitate (i.e., more Productive).
Example 7 . This example illustrates a preferred embodiment of the industrial embodiment of the claimed method. An industrial tubular converter made of tubes with an internal diameter of 32 mm, a length of about 2000 mm, 40 dm 3 of catalyst is charged and ortho-para conversion is carried out under isothermal conditions at T = 21 K and P = 130 kg / cm 2 . As a supply, a "normal" hydrogen containing 25% of parahydrogen is used. Hydrogen containing at least 99% of the para isomer is obtained (the thermodynamically equilibrium concentration of the para-isomer at T = 21 K is 99.7%).
When the industrially used catalyst IR-5-4 (aluminum-nickel extrudates 2 x 3 mm) is loaded with hydrogen at a flow rate of 3000 h -1 / nm 3 hydrogen / m 3 catalyst charge · h, hydrogen is produced with a para-isomer concentration of 99% Leads to a decrease in this indicator to an unacceptable level of less than 99%
When loading the catalyst obtained in accordance with the prototype (a-FeO (OH)), it is possible to increase the power consumption to 25,000 h -1 .
The catalysts according to the invention provide hydrogen with a para-isomer concentration of at least 99% at a flow rate of 40,000 h- 1 (catalyst of Example 3) to 45,000 h -1 (catalyst of Example 2).
CLAIM
A process for the production of parahydrogen by low-temperature conversion of orthohydrogen by contacting hydrogen containing hydrogen fluoride with a hydrated iron (III) catalyst formed in a hexagonal polymorphic modification under cryogenic conditions, characterized in that the process is conducted on a catalyst promoted with hydrated oxides of the general formula FeMe (ON) 3 Or FeMe (OH), where ME is molybdenum, tungsten, chromium, vanadyl and cobalt, at a space velocity equal to (4-4.5) × 10 4 h -1 .
2. A catalyst for producing parahydrogen based on a shaped hydrated iron (III) oxide of a hexagonal polymorph, characterized in that the catalyst further comprises promoters-hydrated oxides and has the general formula FeMe (OH) 3 or FeMe (OH), where Me molybdenum, tungsten , Chromium, vanadyl and cobalt at their quantity (in atomic fractions): Mo (0.0075 0.019), W- (0.00048 0.0011), CR- (0.011 0.025), VO- (0.005 0.012) and Co- (0.012 0.029), the balance of Fe.
2. A process for the preparation of a catalyst for the production of para-hydrogen by adding an equimodium aqueous solution of sodium hydroxide to an aqueous solution of iron (III) chloride to obtain an iron (III) hydroxide sol, forming an iron (III) hydroxide precipitate, settling the precipitate from sodium and chlorine ions by decanting , Drying, shaping and activation in a hydrogen current at 373 423 K, characterized in that a solution of 2-6H hydroxide is used, a solution of sodium hydroxide is added at 288-303 K, and before the precipitate is formed, the iron (III) hydroxide sol is mixed with Sludge, obtained by anodic dissolution of steel, at an atomic ratio of 1 (0.5 1) based on iron.
print version
Date of publication 01.03.2007gg




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.