| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2086502
![]()
METHOD FOR PRODUCING CARBON MATERIAL AND HYDROGEN
Name of the inventor: Chesnokov VV .; Brawlers RA .; Molchanov VV .; Jars GG .; Grave YI
The name of the patentee: Institute of Catalysis SB RAS im.G.K.Boreskova
Address for correspondence:
Starting date of the patent: 1994.04.11
Usage: obtaining ferromagnetic ink, graphite pigments for copying, synthetic and natural rubbers and plastics. The inventive nickel catalyst is charged to the flow reactor is heated in a hydrogen stream to 475 o C, include methane feed. Carry out the decomposition at 550-600 o C. After the fall of the activity of the catalyst is 3-5 times the temperature was raised to 700 o C. Instead of methane and propane can be used to conduct decomposition at 475-500 o C. The ratio of C n H m: H 2 = 3 -10: 1-10 (l / h). Yield carbon 114,8-386,4 g / g reduced catalyst.
DESCRIPTION OF THE INVENTION
The invention relates to the production of carbon, preferably threadlike and hydrogen from the hydrocarbons. Threaded carbon is formed as a coil filament having a diameter of several hundred angstroms to several microns in length. Due to the presence of fine particles of nickel filamentary carbon has ferromagnetic properties and can be used to prepare ferromagnetic ink, graphite pigments for copying, natural and synthetic rubbers and plastics. Along with this, the carbon material can be used in the melting of steels and as a reducing agent in powder metallurgy.
A method of producing hydrogen and carbon by passing methane over massive catalyst (Fe, Co, Ni) at temperatures of 650-720 o C and atmospheric pressure.
The disadvantages of this method are relatively low overall yields of carbon and hydrogen; rapid deactivation of the catalyst (for 0.5-1.0 h); low productivity of the process, solid metal catalyst has a low surface area and accordingly has a low rate of decomposition of methane into carbon and hydrogen.
The invention uses research on the hydrogen influence on the catalytic formation of carbon from hydrocarbons in the nickel. In particular, it was found that in the case of dilution 1,3-butadiene in an inert diluent carbonization nickel catalysts in the form of carbon is deposited film surface and blocking the process of slowing down. Substitution of an inert diluent to reduce the hydrogen at the start of the rate of formation of carbon on nickel because the hydrogen hydrogenates carbon formed on the surface of nickel available reaction medium. As the reaction proceeds, an increase of the rate of carbon. This is due to the formation of carbon nuclei in the inner phase of the intercrystalline cavities aggregates of nickel, nickel dispersion and the formation of filamentous carbon.
The invention aims to increase the yield of the carbon material with hydrogen and a catalyst unit.
The problem is solved as follows.
Reduced nickel-containing catalyst on degradation of paraffins is carried out at temperatures of 475-700 o C in a hydrocarbon medium diluted with hydrogen in a ratio of C n H m: H 2 10.3: 10.1 (l / h) paraffin or decomposition is carried out at temperatures of 475- 600 o C in a hydrocarbon medium with a dilute hydrogen gradual temperature rise after the fall of the catalyst activity is 3-5 times up to 700 o C.
Dilution with hydrogen in the process can be achieved by the hydrogen produced during the decomposition of hydrocarbons in the process of obtaining the target carbon material. This can apply the known methods of recycling of the reaction mixture therein dosing required amount decomposable hydrocarbon.
The amount of hydrogen generated corresponds to the reaction stoichiometry C n H m = nC + 0,5mH 2: CH 4 C + 2H 2 or C 3 H 8 = 3C + 4H 2.
The invention is illustrated by examples and confirmed by the data of the table.
Example 1 A catalyst consisting of 90 wt. NiO and 10 wt. Mg (OH) 2, and the resulting 2-h mechanochemical activation in a planetary centrifugal mill, in an amount of 0,002 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / h) to 550 o C. Then hydrogen is replaced by methane decomposition and reacted at a temperature of 550 o C for 105 hours, and the methane flow of 10 l / h. Weight gain due to carbon catalyst was 3630 wt. relative to the weight of reduced catalyst.
EXAMPLE 2 A catalyst consisting of 90 wt. Ni and 10 wt. Al (OH) 3 and the resulting 30-min mechanochemical activation in a planetary centrifugal mill, in an amount of 0.0026 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / hr) to 550 o C. Then, the hydrogen is replaced by methane and the decomposition reaction is carried out at a temperature of 550 o C for 1.5 hours and the methane flow rate of 10 l / h. Weight gain due to carbon catalyst was 4600 wt. relative to the weight of reduced catalyst.
Example 3 A catalyst composed of 80 wt. NiO, 8 wt. Cu and 12 wt. Mg (OH) 2, and the resulting 2-h mechanochemical activation in a planetary centrifugal mill, in an amount of 0.0018 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / hr) to 600 o C. Then, the hydrogen is replaced by methane and the decomposition reaction is carried out at a temperature of 600 o C for 4.0 hours and the methane flow rate of 3 l / h. Weight gain due to carbon catalyst was 7480 wt. relative to the weight of reduced catalyst.
Example 4 Analogous to Example 3 differs only in the catalyst composition: 75 wt. NiO, 12,5 wt. CuO and 12.5 wt. Mg (OH) 2 and the methane flow rate of 10 l / h. Weight gain due to carbon catalyst was 7670 wt. relative to the weight of reduced catalyst.
Example 5 Same as Example 3 differs only in the catalyst composition: 75 wt. NiO, 12,5 wt. CuO and 12.5 wt. Al (OH) 3 and a reaction temperature of 700 o C. Weight gain due to carbon catalyst was 570 wt. relative to the weight of reduced catalyst.
Example 6 As in example 5 differs only in that the decomposition reaction of methane is carried out in a medium (3 l / h) diluted with hydrogen (5 l / h). Weight gain due to carbon catalyst was 5820 wt. relative to the weight of reduced catalyst.
Example 7 As in example 3 differs only in that the reaction is effected by decomposition of methane medium (3 l / h) diluted with hydrogen (1 l / h). Weight gain due to carbon catalyst was 11480 weight. relative to the weight of reduced catalyst.
Example 8. A catalyst consisting of 75 wt. NiO, 12,5 wt. CuO and 12.5 wt. Mg (OH) 2, and the resulting 2-h mechanochemical activation in a planetary centrifugal mill, in an amount of 0,001 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / h) to 600 o C. Then, the hydrogen consumption is reduced to 3 liters / hr, include 10 l / h and the methane decomposition reaction is carried out at a temperature of 600 o C for 1.5 hours. during this time, the carbon deposition rate decreased by 3 times, and the catalyst for the gain through 3000 was deposited carbon by weight. relative to the weight of reduced catalyst. Further reaction leads to methane decomposition medium (10 l / h) diluted with hydrogen (3 l / h) with a gradual temperature increase from 600 to 630 o C for 8.5 hours. The total catalyst weight gain due to carbon weight was 15,720. relative to the weight of reduced catalyst.
Example 9 As in example 8 differs only in that the decomposition reaction is first carried out at 550 o C methane medium (10 l / h) diluted with hydrogen (4 l / h) for 1 h. The catalyst weight gain due to carbon was 600 wt. relative to the weight of reduced catalyst. Then methane decomposition reaction is carried out in the same reaction medium with gradually raising the temperature from 550 to 630 o C for 6 hours. The total catalyst weight gain due to carbon weight was 15,751. relative to the weight of reduced catalyst.
Example 10 As in example 8 differs only in that the decomposition reaction was conducted first at 570 o C in a medium of methane (10 l / h) diluted with hydrogen (3 l / h) for 1 hour. The weight gain due to carbon catalyst was 900 wt. relative to the weight of reduced catalyst. Then methane decomposition reaction is carried out in the same reaction medium with gradually raising the temperature from 570 to 700 o C. The total catalyst weight gain due to carbon weight was 30,400. relative to the weight of reduced catalyst.
Example 11 As in example 10, differs only in the catalyst composition of 80 wt. NiO, 8 wt. CuO, and 12 wt. Mg (OH) 2. The total gain of the catalyst due to carbon weight was 31400. relative to the weight of reduced catalyst.
Similar studies were carried out with propane.
Example 12. The catalyst composed of 85 wt. NiO and 15 wt. Al (OH) 3 and the resulting 30-min mechanochemical activation in a planetary centrifugal mill, in an amount of 0.0036 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen flow of 20 l / h to a temperature of 500 o C. Then, hydrogen was replaced with propane, the decomposition reaction is carried out at a temperature of 500 o C for 1.5 h, and a propane flow of 5 l / h. Weight gain due to carbon catalyst was 7330 wt. relative to the weight of reduced catalyst.
Example 13. The catalyst composed of 85 wt. NiO and 15 wt. Al (OH) 3 and the resulting 30-min mechanochemical activation in a planetary centrifugal mill, in an amount of 0.00235 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / hr) to temperature of 500 o C. Then, the hydrogen consumption is reduced to 10 l / h of propane were added (10 l / h) and the decomposition reaction is carried out at 500 o C for 6 hours. The catalyst weight gain due to carbon weight was 20,200. relative to the weight of reduced catalyst.
Example 14. The catalyst composed of 85 wt. NiO and 15 wt. Al (OH) 3 and the resulting 30-min mechanochemical activation in a planetary centrifugal mill, in an amount of 0.00115 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / hr) to temperature 475 o C. Then, the hydrogen consumption is reduced to 10 l / h of propane were added (10 l / h) and the decomposition reaction is carried out at 475 o C for 6 hours. The amount of carbon formed 12450 wt. It occurred further reacting with a gradual increase in temperature from 475 to 500 o C for 6 hours. The amount of carbon formed 6670 wt. The total gain of the catalyst due to carbon weight was 19120. o with respect to the weight of reduced catalyst.
Example 15. The catalyst composed of 80 wt. NiO, 8 wt. CuO, and 12 wt. Al (OH) 3 and the resulting 30-min mechanochemical activation in a planetary centrifugal mill, in an amount of 0.00145 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / hr) to 500 o C. Then, the hydrogen consumption is reduced to 5 l / h of propane was added (5 l / h) and the decomposition reaction is carried out at a temperature of 500 o C for 6 hours. The amount of carbon formed 13340 wt. Further carry out the reaction occurred with a gradual increase in temperature from 500 to 700 o C for 40 hours. The amount of carbon formed 38660 w. The total gain of the catalyst due to carbon weight was 52,000. relative to the weight of reduced catalyst.
Example 16. The catalyst composed of 80 wt. NiO, 8 wt. CuO, and 12 wt. Al (OH) 3 and the resulting 30-min mechanochemical activation in a planetary centrifugal mill, in an amount of 0.0014 g was charged in a flow reactor with weights Mc Ben heated for 20-30 minutes in a hydrogen stream (20 l / hr) to 500 o C. Then, hydrogen was replaced with propane (5 l / h) and the decomposition reaction is carried out at a temperature of 500 o C for 1.5 hours after which the catalyst is deactivated and stops the formation of carbon. The quantity of carbon was 4670 wt. relative to the weight of reduced catalyst.
The data provided by the output of the carbon depending on the reaction conditions.
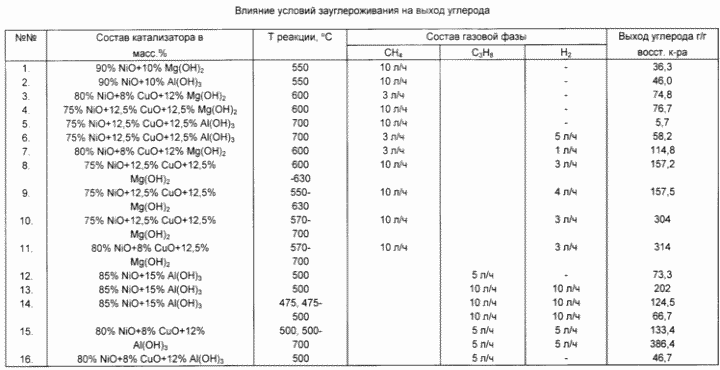
Presented in the table and description of Examples, the invention provides a yield of carbon 114,8-386,4 g / g reduced catalyst, it is possible to use a relatively small amount of catalyst with high specific surface area. The catalyst activity is maintained for a long time, and the generated hydrogen is recycled, significantly increases the productivity of the process.
CLAIM
1. A method of producing hydrogen and carbon material comprising paraffin decomposition nickel-containing catalyst at elevated temperature, characterized in that the decomposition is carried out at 475 700 o C in a medium of gaseous paraffinic hydrocarbon of dilute hydrogen at a ratio of C n H m H January 10 February 3 10 (l / h).
2. A method according to claim 1, characterized in that the decomposition is carried out at 475 - 600 o C and a drop of catalyst activity after 3 5 times the temperature was increased to 700 o C.
3. A method according to claim 2, characterized in that a hydrocarbon is used as the methane and the decomposition is carried out at 550 600 o C.
4. A method according to claim 2, characterized in that as the hydrocarbon is propane and the decomposition is carried out at 475 500 o C.
print version
Publication date 01.03.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.