|
Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2257399
![]()
A METHOD FOR PRODUCING HYDROGEN, ELECTRICITY AND HYDROPROTECTED PRODUCT FROM HYDROCARBON RAW MATERIAL
The inventor's name: Gosselink Johan Willem (NL); GREENEVELD Michiel Yang (NL); NOVAK Andreas Karl (NL); ROVERS Antonius Adrianus Maria (NL)
The name of the patent owner: SHELL INTERNATIONAL RISERCH MAATSHAPPY BV (NL)
Address for correspondence: 103735, Moscow, ul.Ilyinka, 5/2, OOO "Soyuzpatent", Silayeva A.A.
Date of commencement of the patent: 2000.05.09
The invention relates to a process for producing hydrogen, electric power and at least one hydrotreated product from a hydrocarbon feedstock. A process for producing hydrogen, electric power, and at least one hydrotreated product from a hydrocarbon feedstock containing at least a fraction that has the same boiling range or higher than the boiling range of the hydrotreated product to be produced; This method includes the steps of: treating the hydrocarbon feedstock with hydrogen in the presence of a supported catalyst, hydrogen being at least partially obtained from a fraction of the hydrotreated feedstock having a boiling range different from the boiling range of the hydrocarbon feed fraction from which the hydrotreated product , Or at least from a portion of said hydrotreating product, separating the hydrotreated product from the hydrotreated feedstock when the hydrotreated product should be recovered and treating part or all of the remaining hydrotreated feed and the hydrotreated product if it is not recovered in order to produce hydrogen ; And subjecting some or all of the hydrogen that is not used to treat hydrocarbons, processing it to produce electricity; Or subjecting a portion of the hydrotreated feedstock and the hydrotreated product, if it is not recovered, to processing with the production of electricity, and the residue is sent for processing to produce hydrogen. The invention allows simultaneous production of hydrogen, electric power, a and at least one hydrotreated hydrocarbon product.
DESCRIPTION OF THE INVENTION
The invention relates to a process for producing hydrogen, electric power and at least one hydrotreated product from a hydrocarbon feedstock.
The goal of traditional oil refining is the conversion of hydrocarbon feedstocks into one or more useful products. Depending on the availability of raw materials and the desired set of products over time, many processes of hydrocarbon conversion have been developed. Some of these processes are non-catalytic, such as visbreaking and thermal cracking; others, like fluid catalytic cracking (FCC), hydrocracking and reforming, are examples of catalytic processes. Reforming and FCC, although they have very different configurations, are processes with two common features: they are carried out in the presence of a catalyst and their purpose is to produce from the raw materials used hydrocarbon materials having different compositions.
Usually, special attention is paid to the production of one or more valuable hydrocarbon products. For example, reforming and FCC are processes that are specifically aimed at producing large amounts of gasoline as the main product (usually in the FCC process, and some of the lower olefins are formed, and during the reforming process some hydrogen is formed), while hydrocracking is aimed at obtaining , Depending on operating conditions, naphtha or medium distillates.
Taking into account the value of hydrocarbons, especially liquid hydrocarbons as transport fuel components, it will be clear that maximizing the production of a single hydrocarbon product, whether it is gasoline or diesel fuel, or optimizing the list of products in the case where it is necessary to obtain two or more valuable products is very Important for the design of refineries, both for newly constructed plants, and for the modernization of existing installations, or the expansion of the number of existing plants. Therefore, the formation of by-products (such as lower olefins or hydrogen) is usually minimized, or, when there is a specific need for such products, it is always considered in the context of not too strong a reduction in the yield of the main products.
It is known in the literature that products similar to lower olefins and hydrogen can be obtained from special sources, which usually have the nature of hydrocarbons. However, in such processes, the goal is to maximize the production of such products, and therefore, other hydrocarbon products are not formed or actually formed at the same time.
For example, a known method for producing hydrogen is gasification of methane or electrolysis of water. In such processes valuable liquid hydrocarbons are not formed. Lower olefins like propylene and butylene are conveniently obtained by (catalytic) dehydrogenation of the corresponding alkanes (propane and butane). And in the case of these processes, valuable liquid hydrocarbons are not formed either.
In many industrial centers there are installations that operate in an additional mode. For example, the hydrogen required for the hydrogenation processes is formed as a result of the isolated gasification process, with olefins that are a suitable feed, for example for polymerization processes that can be carried out in the same or a neighboring center, will be produced in an FCC plant in which Still produced gasoline as the main product.
As for the production of electricity, it is known that electricity (as the main product and in many cases the only product) can be produced from a variety of organic raw materials, from coal and natural gas to oil or residual materials. And in this case it can be understood that liquid hydrocarbons will not be formed in such a process.
EP-A-435736 proposes to generate electricity by using a fuel cell to which hydrogen is supplied by improved fuel reforming, subjecting low-boiling petroleum fuel to cracking and desulfurizing treatment at a pressure not exceeding 10 kg / cm 2 (1 MPa) in the presence of Of a non-carried zeolite catalyst. It turned out that under the conditions described in EP-A-435736, significant amounts of unwanted aromatic compounds are formed, even when working under pressure as low as 0.5 MPa.
However, there is a need to provide the ability to produce hydrogen, electricity and one or more (liquid) hydrocarbon products in a combined process. In particular, there is a need to provide flexibility in relation to the relative quantities of the three main products (hydrogen, electricity and (liquid) hydrocarbon products) that will be produced. In regions where support facilities and / or additional production centers are not available, the combined way in which three key products are formed, in fact, can be the only possible option. In addition, it would be highly desirable if such a combined process could be carried out both on a large scale and on a small scale, or could be used as additional support for existing plants.
In the present invention, it has been found that it is possible to combine unusual production targets, such as hydrogen, electricity, as well as at least one hydrotreated hydrocarbon product. Moreover, it has been found that, depending on local needs, the product list (for the three key products) can be very flexible, which provides very wide application possibilities.
Therefore, the present invention relates to a process for producing hydrogen, electric power, and at least one hydrotreated product from a hydrocarbon feedstock containing at least a fraction that has the same boiling range or higher than the boiling range of the hydrotreated product to be produced ; This method includes the steps of: treating the hydrocarbon feedstock with hydrogen in the presence of a supported catalyst, hydrogen being at least partially obtained from a fraction of the hydrotreated feedstock having a boiling range different from the boiling range of the hydrocarbon feed fraction from which the hydrotreated product , Or at least from a portion of said hydrotreating product, separating the hydrotreated product from the hydrotreated feedstock when the hydrotreated product is to be isolated and treating part or all of the remaining hydrotreated feed and the hydrotreated product if it is not recovered in order to produce hydrogen ; And subjecting part or all of the hydrogen that is not used to treat hydrocarbons, processing it with electricity; Or subjecting a portion of the hydrotreated feedstock and the hydrotreated product, if it is not recovered, to processing with the production of electricity, and at least part of the residue is sent for processing to produce hydrogen.
Depending on the specific requirements of the infrastructure in which the method of the present invention will be implemented, operators will have a choice within the range of products in the production direction of all three main products (hydrogen, electricity and hydrotreated product) or direct the process to the production of two products, or even to Production of a single product.
In the case where hydrogen and electricity are the desired products and at the same time there is no need to produce a hydrotreated product, the total amount of the hydrocarbon material (both remaining after the hydrogen treatment and the hydrotreated product) can serve as a raw material for the subsequent production of hydrogen and electricity.
A preferred feature of the process according to the invention is that at least the amount of hydrogen and electricity that is necessary to meet the internal needs of the process in the sense of hydrogen to be used in the hydrogen treatment and the electricity that is required to start the process from the point View of material security. Of course, it is possible to supply part of the hydrogen and / or part of the electricity required in this method directly from external sources, provided that they are available or can be made available.
Having provided these internal needs, the oil refiner can still make a choice to optimize production in the sense of hydrogen or electricity as the main product. In the case where hydrogen is required as the main product, only the amount of electricity needed to operate the plant will be produced, and in the case where the main focus is on the production of electricity as the main product, only the amount of hydrogen needed to satisfy Domestic demand (hydrogen, which will be used in processing products with hydrogen), and the rest of the hydrogen produced will serve as raw material for electricity generation.
The various hydrocarbon feedstocks that can be conveniently used in the process of the present invention have boiling limits in the range from the boiling point of about ambient temperature to the boiling point temperature of about 650 ° C. measured under normal conditions (temperature 20 ° C, pressure 1 atmosphere). It can be understood that the feedstock that can be used in the process of the present invention does not need to have a boiling point characteristic (a distillation curve) that covers the entire above-mentioned range. And it is advantageous to use a raw material having such a boiling range that the temperature at which 90% of the material is boiled (i.e., the temperature at which 90% of the feedstock is distilled during distillation) is between 400 and 600 ° C. Preference is given to the feedstock, 90% of which is boiled between 450 and 600 ° C. Good results can be obtained by using raw materials, 90% of which is boiled in the range from 475 to 550 ° C.
Examples of feedstocks that can be suitably used are naphtha, kerosene and various kinds of gas oils, such as atmospheric gas oil and vacuum gas oil. In addition, recycle gas oil can be conveniently used. It is possible to use raw materials not only mineral, but also synthetic origin. Synthetic or semi-synthetic feedstocks are preferred for use because of low sulfur and / or nitrogen content, since there is no need for sulfur and / or nitrogen removal processes that are part of the product quality improvement unit for such raw materials.
Hydrocarbon products derived from syngas using the so-called Fischer-Tropsch process are a very valuable raw material for the process of the present invention, since sulfur and / or nitrogen purification processes can be excluded for such raw materials and appropriate disposal equipment These impurities.
It is possible that the hydrocarbon feedstock that can be used in the process of the present invention and contains substances having a boiling point below ambient temperature. Such substances can be present in the processed feedstock or can be added to such raw materials. This refers to the presence of lower hydrocarbons or hydrocarbon fractions, such as liquefied petroleum gas.
It is advantageous to use a feedstock that contains between 5 and 40% by weight of a material that has the same boiling range or higher than the boiling range of the hydrotreated product to be recovered from the hydrotreated feedstock (and, as appropriate, can be used, at least Partly as a raw material for the production of hydrogen, in order to use it for the internal needs of the process according to the invention or as the final hydrotreating product). Preferably, the raw material is a material in which from 5 to 40% by weight is boiled in an interval higher than the boiling point of the hydrotreated product that will separate from the hydrotreated feed.
In addition, it is possible to process raw materials that contain sulfur compounds. Typically, the sulfur content will not exceed 5% by weight, and preferably the sulfur content does not exceed 3% by weight. Most preferred is a feedstock with an even lower sulfur content or no sulfur at all.
It will be understood by those skilled in the art that it will be necessary to introduce hydrogen from an external source, at least in connection with the start-up of the process in accordance with the present invention. For example, you can use the hydrogen available in the storage tank. Part or all of the amount of hydrogen that will be consumed in the hydrotreating step in the process of the present invention will be produced in a hydrogen production unit that is part of the production chain.
Treatment of the feedstock with hydrogen in the presence of a supported catalyst according to the process of the present invention is essentially a treatment for changing the composition of the feed, i.e., a hydrogenation conversion process (hydrotreating). The rigidity of the hydrotreating regime depends on the nature of the hydrotreated product that is desired to be obtained, and on the nature of the feedstock to be treated with hydrogen.
The hydrotreating process according to the present invention can conveniently be carried out in a temperature range between 100 and 550 ° C, preferably between 250 and 450 ° C. Pressure can be used up to 400 atmospheres (40 MPa), but preference is given to pressure in the range between 10 and 200 atmospheres (1-20 MPa).
In the case where the aim of the process according to the present invention is to produce kerosene and / or gas oil as hydroprocessed products that will at least partially be recovered and not used for other purposes (i.e., hydrogen and electricity are obtained mainly from the remaining hydrotreated Raw materials), treatment with hydrogen, in fact, will be a hydrocracking process in which heavier fractions of raw materials will be converted in the mode of operation of hydrocracking.
At the same time, in the process according to the present invention, it is necessary to produce at least a portion of the hydrogen used in the hydrogen treatment. Therefore, catalysts that are able to convert not only that portion of the feedstock that allows the hydrotreated product to be made, but also convert other constituent parts of the feedstock to such an extent that the remaining hydrotreated feedstock is a good starting material for the production of hydrogen, is therefore preferred for use. In other words, preference is given to catalysts, which, when used, produce a large number of low-boiling materials (except for the hydrotreated product).
Examples of supported catalysts that can be used in the hydrogen treatment process in accordance with the process of the present invention are zeolite-containing catalysts having a tendency to excessively deep, from a traditional point of view, cracking of hydrocarbon compounds (cracking, if possible, only those feedstock fractions from The desired products of cracking are obtained, however, it is desirable to keep the feedstock as much as possible, or at least the liquid materials that will remain, and thus minimize the formation of gaseous substances. In the process of the present invention, it is advantageous to use hydrocracking catalysts on which, in addition to the desired products, a significant amount of low boiling products can be obtained which, from the standpoint of conventional hydrocracking, are not at all preferred. For example, such catalysts can be based on zeolite beta, zeolite Y, ZSM-5, erionite and chabazite. It will be understood by those skilled in the art that which zeolite material and which particular metals possessing hydrocracking activity can be used. At the same time, it is necessary to take into account that catalysts giving a higher yield relative to light products are preferred because such products reduce the rigidity of the regime in that part of the process that is directed at hydrogen production. Examples of suitable catalysts include zeolite beta containing one or more Group VI metals and / or one or more Group VIII metals. Examples of Group VI metals include Mo and W. Examples of Group VIII metals include Ni, Co, Pt and Pd. Suitable catalysts contain from 2 to 40% by weight of Group VI metals and / or 0.1 to 10% by weight of Group VIII metals.
Examples of suitable support materials are alumina, silica, aluminosilicate, magnesia, zirconia and mixtures of two or more such carriers. Alumina is the most preferred carrier material, optionally in combination with an aluminosilicate.
In addition, combinations of two or more catalysts can be suitably employed. Examples of combinations of catalysts include so-called block catalyst beds, which include the use of different layers filled with (different) catalytic materials. The choice of particular combinations of catalyst layers will depend on the envisaged process regime, which is known to those skilled in the art.
A catalytic system is employed that allows at least 50 wt.%, Preferably at least 65 wt.%, Of the hydrocarbon feedstock to be converted in one pass, which has a boiling range of the same or higher as compared to the boiling range of the hydrotreated product .
In addition, it is possible that the composition of the feedstock and the set of desired products (hydrogen, and electricity, and a hydrotreated product that can be used, partially or completely, to produce hydrogen and electricity) are interrelated in such a way that there is no need for such treatment with hydrogen, Which would lead to the need to reduce the boiling range of the hydrotreated product. In other words, the fraction may be present in the raw material, which already has the intended properties of the hydrotreating product. This could mean that special attention in processing with hydrogen should be given to the composition of the remaining hydrotreated feedstock (remaining after the separation of the corresponding hydrotreating product). Such a treatment will, in essence, be the saturation of the olefinic and / or aromatic compounds present in the feed. Optionally together with the removal of compounds containing heteroatoms, which is possibly accompanied to some extent by hydrocracking.
When treated with hydrogen, a catalyst containing zeolite beta as an active ingredient is used.
Catalysts that can be conveniently used in such conditions include traditional hydrotreating catalysts. Examples of such catalysts include hydrotreating catalysts based on alumina, silica or aluminosilicate containing one or more Group VI metals and / or Group VIII metals. Examples of Group VI metals include Mo and W. Examples of Group VIII metals include Ni and Co. Suitable catalyst systems contain Co and Mo, or Ni and Mo, on alumina or amorphous aluminosilicate.
In the case where oil refiners choose only the production of hydrogen and / or electricity as the final product, the entire hydrotreated product together with the hydrotreated feedstock can be used as a raw material for the production of hydrogen and electricity. At least some of the hydrogen produced can be used in the process of the present invention in order to satisfy at least some of the process needs in connection with the processing conditions of hydrogen; The rest, i. Hydrogen not used in the hydrotreating stage, at least in part, can be used to generate at least a portion of the electricity required in the process, and the remainder can either be considered as an end product or, depending on the local infrastructure , It will be transformed, at least in part, into electricity.
An important embodiment of the method according to the present invention is an embodiment in which the hydrogen treated kerosene is a hydrotreated product that will be recovered from the process, hydrogen is produced in an amount sufficient to meet the internal needs of the process and the electricity is produced not only for use in Process, but also available for export to the local network.
The remaining hydrotreated feed, optionally in combination with a part or even the entire hydrotreated product, in the case where there is no direct yield for this product, can be subjected to treatment in order to produce hydrogen, with at least a portion of this hydrogen being used to meet the demand In the hydrogen of the process according to the invention, or a part thereof is subjected to a treatment for producing electric power, while the residue is subjected to treatment in order to obtain hydrogen.
Since some hydrogen may already be present in the feedstock for a hydrogen producing plant, it may be beneficial that hydrogen is separated from the hydrotreated feed and from the hydrotreated product if the latter is not recovered prior to the hydrogen production stage. This can be conveniently done if one involves the remaining hydrotreated feed in the separation process on the membrane, which allows hydrogen to pass through and at the same time retains heavier molecules.
Those skilled in the art will recognize the membranes that can be used and the mode of operation thereof.
Many methods are known from the art by which hydrogen can be obtained from a hydrocarbon feedstock. Those skilled in the art are aware of such methods and modes of operation. A suitable method is catalytic (partial) oxidation. Other convenient methods are vapor methane reforming and catalytic dehydrogenation of lower alkanes such as propane or butane.
Preferred hydrogen production systems can be developed by combining partial catalytic oxidation and water gas conversion, wherein in the latter reaction the carbon monoxide formed together with hydrogen in the partial catalytic oxidation process in the presence of water (is steam under process conditions) is converted to Hydrogen and carbon dioxide. The overall result of combining the reactions of catalytic oxidation and the conversion of water gas is that the hydrocarbon material is converted to hydrogen and carbon dioxide.
Generally, the combined process of catalytic oxidation and water gas conversion can be operated with an efficiency of at least 50%, based on the hydrogen produced, preferably at an efficiency of at least 65%, based on the hydrogen produced (without taking into account the hydrogen present In hydroprocessed raw materials).
Suitable catalysts for the partial catalytic oxidation process according to the process of the present invention include one or more Group VIII metals of the Periodic Table of the elements which are supported on a carrier. Examples of suitable metals include rhodium, iridium and ruthenium, and a combination of two or more such metals. It is particularly convenient to use carriers having a high degree of tortuosity. Suitable process conditions include the use of an oxygen: carbon molar ratio in the range between 0.30 and 0.80, preferably between 0.45 and 0.75, and most preferably between 0.45 and 0.65; Temperatures between 800 and 1200 ° C, in particular between 900 and 1100 ° C, using a gas flow rate in the range between 100,000 and 10,000,000 liters / (kg · h), preferably between 250,000 and 2,000,000 liters / (kg · h) .
An advantage of the process according to the present invention is that when hydrogen is obtained as the main product, at the same time an appreciable amount of carbon dioxide is generated which can be used for industrial purposes such as increasing the recovery of oil or for heating in the case of an appropriate infrastructure (Such as housing and communal services or greenhouse agriculture).
The method of the present invention is for generating electricity. This can be achieved as the final stage of the method according to the present invention, when the electric power should be produced from the hydrogen already produced, but it can be obtained from a portion of the hydrotreated feed and the hydrotreated product if it is not recovered, while the rest of it is processed with Hydrogen production. During normal operation, at least sufficient electricity will preferably be produced in order to satisfy the requirements from an operational point of view. Again, in this case, it can be understood that during the start-up of the process according to the invention, there will be a need for electricity from external sources.
Electric power can be produced in various ways that are known to those skilled in the art, and such methods are known in which hydrogen is converted into electricity. An example of a method that can be used to convert hydrogen to electric power is a fuel cell. When the fuel cell operates, water (steam) will be obtained which can conveniently be used, at least as part of the steam required for the operation of the water gas conversion process, when it is combined with the partial catalytic oxidation process to produce hydrogen, according to the method According to the invention.
Preferably, the fuel cell operates in such a way that at least the amount of electric power necessary to satisfy the internal needs of the method according to the invention is produced therein. In those cases where there is no need to produce more hydrogen than is necessary to satisfy (part or all) internal demand for the process according to the invention, the focus is either on optimizing the production of the hydrotreated product as the transport fuel directly entering the market (and thus producing a minimum The amount of hydrogen and electricity that is required for operation with accumulation) or to optimize electricity production, taking into account the market situation with the demand for the hydrotreated product. In the ultimate situation, it is possible that the entire hydrotreated product, together with all the remaining hydrotreated feedstock, is converted to hydrogen, which then becomes electricity, which then becomes the only exported product of the integrated process (after satisfying the domestic needs for hydrogen and electricity discussed above).
The efficiency of the fuel cell to be used in the process of the present invention should be at least 30% based on the hydrogen feedstock. Preference is given to process conditions that provide a degree of conversion of the incoming hydrogen of at least 40%, and most preferably 50%.
Since a raw material containing up to about 5% by weight of sulfur can be used in the process of the present invention, treatment with hydrogen can lead to the formation of hydrogen sulfide. It can be understood that in such situations an additional technological stage will be needed to remove hydrogen sulphide from the hydrotreated feedstock and to convert hydrogen sulphide to sulfur. In the event of a pressure drop prior to the separation of the hydrotreated product, the hydrogen sulphide will preferably be removed and it can be sent to a subsequent process unit, such as an SCOT installation, or if the hydrogen concentration is sufficiently high, it can be fed directly to the Claus unit. Those skilled in the art are aware of such technological installations and their operating modes.
Various embodiments of the method according to the present invention can be schematically illustrated with the aid of the drawing.
In the drawing, an embodiment is shown in which the sulfur containing feedstock is processed so as to produce at least one hydrotreated product that will be recovered as a product for sale on the market together with the hydrogen produced for use in the process of the invention, But also for export.
The feedstock is introduced via line 1 to a hydrotreating unit 10 in which it is subjected to hydrogen treatment in the presence of a supported catalyst which is introduced into line 1 through line 9. From the hydrotreating unit 10, the hydrotreated feedstock is supplied via line 2 to a separation unit 20 in which a hydrotreated The product that is withdrawn via line 3 and a post hydrotreating stream containing hydrogen sulphide will be withdrawn via line 4 to the hydrogen sulfide removal unit 30. From the plant 30, a stream containing hydrogen sulfide will be withdrawn via line 5 to a sulfur recovery unit Not shown) for the production of sulfur, and the post-hydrotreated stream with a low hydrogen sulphide content that can be supplied via line 6a to the hydrogen evolution unit 35 (or, in the case where hydrogen is not released in this process section, is sent directly along line 6 (6a + 6b) to hydrogen production unit 40) from which hydrogen is liberated, is sent back along line 36 to line 1 as part of the hydrogen required for hydrocracking unit 10, and the remaining stream of hydrotreated feed with reduced hydrogen sulphide content (and optionally hydrogen) is fed via line 6b to a hydrogen production unit 40. In the case where this plant has a partial catalytic oxidation stage and a water gas conversion step, water (or steam) through line 11 will be supplied to this water gas conversion step. Carbon dioxide will be withdrawn from line 8, and the hydrogen produced will be fed back to the hydrotreating unit 10 via lines 7 and 9 (optionally together with hydrogen via line 36), while the amount of hydrogen needed to produce part or all of the electricity required with The engineering support viewpoint is taken via line 10 to the electricity generation device 50 (preferably the fuel cell). The electric power received in the device 50 will be fed back to the relevant portions of the process chain (not shown) via line 12, and the water formed in the electricity generation device 50 can be fed back to the hydrogen production unit 40 via line 11.
Two other process variants are shown in the drawing. In the case where it is desired to produce an excess of hydrogen (i.e., more hydrogen than necessary for the operation of the hydrotreating unit 10 in the appropriate mode), the ratio between the hydrotreated product obtained and the hydrotreated feedstock with reduced hydrogen sulphide content will vary such that in the hydrogen production unit 40 An additional amount of hydrogen can be obtained that can be taken via line 13. Similarly, in the case where additional power generation is desired (i.e., more electricity than necessary to meet the operational requirements for the envisaged method), the amount of hydrogen produced (and, accordingly, the production of the hydrotreated Product) will be modified in such a way as to ensure the production of excess electricity that will be taken via line 14.
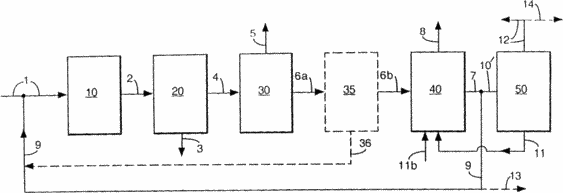
In the drawing, a further variant of the process in which the sulfur-containing feedstock is processed in such a way that all of the hydrotreated feedstock (including the fraction that is recovered as the hydrotreated product in the embodiments of FIG. 1) can be used to produce excess hydrogen and excess electricity , That is, a method in which, with the exception of sulfur and carbon dioxide, the final products are only hydrogen and electricity. In this embodiment, the hydrotreated product normally recovered via line 3 is now fed along with the hydrotreated feed through line 4 to the hydrogen sulfide removal unit 30, and then the subsequent steps are similar to those described in the drawing.
A further embodiment is that raw materials that do not contain sulfur (that is, synthetic or semi-synthetic raw materials or raw materials that have already been hydrodesulfurized) are used. In such an embodiment, it is no longer necessary to separate the hydrotreated feed containing hydrogen sulphide (or supply all the hydrotreated feed to the (optional) hydrogen evolution unit); This means that the method that is schematically shown in FIG. 1 is now operated without the use of a hydrogen sulfide removal unit 30.
INDICATIVE EXAMPLES
The method of the present invention can be illustrated by the following representative examples.
Example 1
Hydrocarbon feedstocks having a boiling point of 121 ° C. and a boiling point of 90% of the material of 533 ° C. and a sulfur content of 0.02% by weight are passed (in an amount of 10 tons / day along with 1.5 ton / day of hydrogen, Which characterizes the hydrogen / feed ratio) above the beta-zeolite catalyst supported on alumina in the hydrotreatment unit 10 under conditions such that in one pass the feedstock is converted to 90% by weight into a material with a lower boiling point. As the product, 85 wt.% Of the hydrotreated product (including naphtha, kerosene and gas oil) can be obtained, based on the hydrocarbon feed, while the remaining hydrotreated feed can be fed to the hydrogen sulfide removal unit. After separation of the hydrogen present in the hydrotreated feedstock (and its return to the feedstock, which can be used as part of the hydrogen required for hydrocracking), after leaving the hydrogen sulfide removal unit, 15% by weight, based on the hydrocarbon feed, can be fed to a hydrogen production unit 40 (Contains a partial catalytic oxidation unit in combination with a water gas shift reactor) into which steam can be added in an amount of 2.1 tons per day. Under the prevailing conditions, 325 kg / day of hydrogen can be obtained (together with the formed 5.1 t / day of carbon dioxide). Of this amount of hydrogen produced in the hydrogen production unit, 125 kg / day can be used as a feedstock in the electricity generation device 50 (preferably a fuel cell) that will allow this amount of hydrogen to be converted, with an efficiency of about 40%, to electricity 70 kW), which can be fed to the appropriate parts of the process chain, while 200 kg / day of hydrogen to compensate for the consumption of hydrogen in the hydrotreating stage is directly fed to the hydrotreating unit (together with hydrogen, which is already separated in the hydrogen separation unit). In this method, 5.1 tons / day of carbon dioxide, and 900 kg / day of water vapor (which can be used in a hydrogen production plant) can be obtained simultaneously.
Example 2
The hydrocarbon feedstock described in Example 1 can be treated as described in Example 1 in a hydrotreating unit 10 (with a hydrogen consumption of 200 kg of hydrogen per day) under conditions that ensure the degree of conversion of the feed per pass into the low temperature material Boiling point of 90 ° C. Under these conditions, 45% by weight of kerosene and gas oil can be obtained as the hydrotreated product. After removal of hydrogen sulfide and separation of 55% by weight of hydrogen based on the feed of the starting material, the product containing naphtha and low boiling point materials can be sent to a hydrogen production unit, into which 7 tons of steam per day is fed. Under normal conditions, it is possible to obtain 1.1 tons of hydrogen per day, of which 125 kilograms per day are directed to the power generation plant to produce 70 kilowatts of electricity, while 775 kilograms per day of hydrogen are available for export, the remainder can be used to meet Part of the hydrotreating requirements in the corresponding plant 10. In this method, 17 t / day of carbon dioxide and 900 kg / day of steam can be produced simultaneously (which can be used in a hydrogen production unit).
Example 3
The hydrocarbon feedstock described in Example 1 can be treated with hydrogen in the presence of a supported catalyst as described in Example 1 in an apparatus for producing electricity for export. When hydrogen is consumed equal to 300 kg of hydrogen per day, and under conditions where the degree of conversion of the feed per pass is 90%, 15% by weight of kerosene and gas oil can be obtained based on the feedstock. After removing hydrogen sulphide and separating the recycled hydrogen by 85% by weight based on the feed of the feedstock, the product containing naphtha and the low boiling point material can be sent to a hydrogen production unit, into which 11 tons of steam per day are fed. Under normal conditions, it is possible to obtain 1.75 ton / day of hydrogen, as well as 27 tons / day of carbon dioxide. The plant for producing electricity can be operated in such a way as to obtain 820 kW of electricity, of which 70 kW can be used to satisfy the power supply in the production line, and 750 kW can be offered to the local network. In this variant, 10.3 t / day of water will be produced simultaneously.
Example 4
The hydrocarbon feedstock described in Example 1 can be treated with hydrogen in the presence of a supported catalyst as described in Example 1 in an installation designed to produce excess hydrogen as the main product and electricity to satisfy the material support of the process, and at the same time not A hydrotreated final product is produced. With a hydrogen consumption of 400 kg per day and a conversion rate of 90% of the feedstock, a hydrotreated feedstock can be obtained which, after removal of hydrogen sulfide and separation of hydrogen, can be completely directed to a hydrogen production plant, into which 13 tons of steam per day . In this plant it is possible to produce 2.05 tons of hydrogen per day, of which 1.5 tons per day can be available for export, while 125 kilograms per day should be sent to an electricity production plant in order to obtain the required amount of electricity, Can be fed to a hydrotreating unit to meet the hydrogen demand in said plant. In this method, 32 tons / day of carbon dioxide and 900 kg / day of steam can be produced simultaneously (which can be used in a hydrogen production unit).
Example 5
The hydrocarbon feedstock described in Example 1 can be treated with hydrogen in the presence of a supported catalyst as described in Example 1 in an installation designed to produce excess electricity as the main product together with hydrogen to meet process needs, and at the same time not A separate hydrotreated product is produced. With a hydrogen consumption of 400 kg per day and a conversion rate of 90% for the feedstock that can be obtained using a beta-type zeolite catalyst, a hydrotreated feedstock is formed which, after removing hydrogen sulfide and separating the recycled hydrogen, can be completely directed to the plant For the production of hydrogen, into which it is necessary to submit 13.5 tons of steam per day. In this plant, it is possible to produce 2.1 tons of hydrogen per day, of which some amount to meet the internal demand of the process in hydrogen can be fed to the hydrotreating unit (taking into account the amount of hydrogen that has already been released during the separation process prior to hydrogen production). The rest (large) part of the hydrogen produced can be directed to the fuel cell, in which 920 kW of electricity can be produced. In this embodiment, 32 t / day of carbon dioxide (without the hydrogen production unit) and 12 t / day of water can simultaneously be produced.
Example 6
The hydrocarbon feedstock described in Example 1 can be treated with hydrogen in the presence of a supported catalyst as described in Example 1 in an apparatus for producing all three basic products (hydrotreated product, hydrogen and electricity) according to the present invention. In the same manner as described in Example 2, as a hydrotreated product, 45% by weight of kerosene and gas oil can be obtained. The product, 55% by weight, based on the feed of the feedstock, containing naphtha and low boiling point materials, can be sent to a hydrogen production unit, into which 7.1 tons of steam per day is fed. Under normal conditions, it is possible to produce 1.1 tons of hydrogen per day, of which 125 kilograms per day should be spent on the production of electricity consumed for process engineering, 125 kilograms per day of hydrogen can be used for export, and the rest of the hydrogen produced in the plant for Production of hydrogen can be supplied to an electricity production plant to produce 425 kW / day of electricity (taking into account the hydrogen demand for the hydrotreatment unit in combination with the amount of hydrogen that has already been released during the separation to the hydrogen production stage). In this embodiment, 17 t / day of carbon dioxide, a and 5.6 t / day of steam (which can be used in a hydrogen production unit) can be simultaneously produced.
Example 7
The hydrocarbon feedstock described in Example 1 can be treated with hydrogen in the presence of a supported catalyst as described in Example 1 in an apparatus for producing a hydrotreated product and an excess of electricity as well as an absorbed hydrogen. With a hydrogen consumption of 150 kg per day and a conversion rate of 65% for the feedstock that can be obtained using a beta-type zeolite catalyst, 72% by weight of kerosene and gas oil can be obtained as the hydrotreated product. The product, 28% by weight, based on the feed of the feedstock, containing naphtha and low boiling point materials, can be sent to a hydrogen production plant, into which 3.6 tons of steam per day are fed. Under normal conditions, it is possible to obtain 550 kg / day of hydrogen, of which some amount to meet the internal demand of the process in hydrogen can be fed to a hydrotreatment unit, 125 kg / day should be directed to the production of electricity consumed for process engineering and the rest of the hydrogen can be To supply to an electricity production plant in order to obtain electricity (150 kW). In this process, 8.9 t / day of carbon dioxide, a and 2.9 t / day of water vapor (which can be fed to a hydrogen production unit) can be simultaneously produced.
Example 8
The hydrocarbon feedstock described in Example 1 can be treated with hydrogen in the presence of a supported catalyst as described in Example 1 in an apparatus for producing a hydrotreated product, hydrogen and electricity (in excess of the amount consumed for process engineering) , In which hydrocarbons produce both hydrogen and electricity. With a hydrogen consumption of 300 kg / day and a conversion rate of 90% for the feedstock that can be obtained using a beta-type zeolite catalyst, 15% by weight of kerosene and gas oil can be obtained as the hydrotreated product. The material, 28% by weight based on the feedstock feed, containing naphtha and a fraction with a reduced boiling point, can be used for the production of hydrogen and electricity. Accordingly, 17% by weight of this material can be directed to a hydrogen production unit, into which 2 tons of steam per day are fed. Under normal conditions, it is possible to obtain 300 kg / day of hydrogen to meet the internal demand of the process, at the same time, it is possible to produce 4.5 t / day of carbon dioxide. Accordingly, 83% by weight of the total amount of naphtha and the fraction with reduced boiling point can be fed to an electricity production plant to generate electricity (1820 kW), from which typically 70 kW can be used to meet process needs, and 1,750 kW are available for export. In this embodiment of the process, 22.5 t / day of carbon dioxide can be simultaneously produced.
Example 9
The hydrocarbon feedstock described in Example 1 can be treated with hydrogen in the presence of a supported catalyst as described in Example 1 in an installation for producing hydrogen and electric power as products that are formed from a hydrotreated feedstock (i.e. in this embodiment a hydrotreated Product is not allocated). With a hydrogen consumption of 400 kg / day and a conversion rate of 90% for the feedstock that can be obtained with the beta-type zeolite catalyst, the resulting hydrotreated feed can be used after removal of hydrogen sulfide and separation of hydrogen to produce hydrogen therefrom And electricity. Accordingly, 24% by weight of this material can be directed to a hydrogen production unit, into which 2.55 tonnes of steam per day are fed. Under normal conditions, it is possible to obtain 400 kg / day of hydrogen to meet the internal demand of the process, at the same time, 6 tons / day of carbon dioxide can be produced. Accordingly, 76% by weight of the hydrotreated feedstock can be fed to an electric power plant to generate electricity (2120 kW), from which typically 70 kW can be used to meet the process requirements, and 2050 kW are available for export. In this embodiment of the process, 26 t / d of carbon dioxide can be simultaneously produced.
CLAIM
A process for producing hydrogen, electric power, and at least one hydrotreated product from a hydrocarbon feedstock that has a boiling range such that its boiling point of 90% of the material is between 400 and 600 ° C containing at least a fraction Which has a boiling range of the same or higher in comparison with the boiling point range of the hydrotreated product to be obtained, characterized in that the hydrocarbon feedstock is treated with hydrogen under a pressure in the range of 10 to 400 atmospheres in the presence of a supported catalyst, At least in part, is obtained from a fraction of the hydrotreated feedstock that has a boiling range different from the boiling range of the hydrocarbon feedstock fraction from which the hydrotreated product will be obtained, or at least a portion of said hydrotreated product, the hydrotreated product is separated from the hydrotreated product Of the raw material when the hydrotreated product separates and part or all of the remaining hydrotreated feedstock is treated and the hydrotreated product, if not recovered, to produce hydrogen, and subjected to a portion or all of the hydrogen that is not used for hydrocarbon treatment, processing with electricity generation, or subjected to A portion of the hydrotreated feedstock and the hydrotreated product, if it is not recovered, is recycled to produce electricity and at least a portion of the residue is subjected to processing to produce hydrogen.
2. A method according to claim 1, characterized in that a hydrocarbon feedstock having a sulfur content of not more than 5% by weight, preferably less than 3% by weight, is used.
3. A method according to claim 1 or 2, characterized in that a hydrocarbon feedstock containing a fraction in an amount between 5 and 40% by weight, which has a boiling range of the same or higher than the boiling range of the hydrotreated product, is used Will receive.
4. The process of claim 3, wherein the hydrocarbon feed comprises a fraction in an amount between 5 and 40% by weight, which has a boiling point higher than the boiling point of the hydrotreated product.
5. The process of claim 4, wherein kerosene and / or gas oil are recovered from the hydrotreated feedstock as a hydrotreated product (s).
6. The method of claim 1, wherein part or all of the remaining hydrotreated feed and the hydrotreated product, if not recovered, is brought into the catalytic oxidation process in which hydrogen and (di) carbon monoxide is formed.
7. The process of claim 6, wherein the catalytic oxidation process includes a partial catalytic oxidation process and a water gas shift process.
8. A method according to claim 6 or 7, characterized in that hydrogen not used in the hydrotreating step is at least partially used for generating electricity by supplying hydrogen to a fuel cell operating in the production mode of electricity and water (water vapor).
9. A method according to claim 8, characterized in that excess electricity is produced from excess hydrogen in excess of that required for the process.
10. The method of claim 8, wherein at least a portion of the steam required for the hydrogen production unit is provided by the fuel cell.
11. The method of claim 7, wherein kerosene and / or gas oil, hydrogen, carbon dioxide and electric power are obtained only from raw materials not differing from the hydrocarbon feed and water used in the water gas conversion step.
12. The method of claim 1, wherein hydrogen sulphide generated by treating the hydrocarbon feedstock with hydrogen is converted to elemental sulfur.
13. A process according to claim 1, characterized in that a catalyst is used to convert at least 50% by weight, preferably at least 65% by weight, of a hydrocarbon feedstock that has a boiling range of one The same or higher in comparison with the boiling range of the hydrotreated product.
14. The method of claim 13, wherein the hydrogen treatment uses a catalyst containing zeolite beta as an active ingredient.
15. The process of claim 14, wherein the zeolite beta catalyst allows one to convert at least 90% by weight of the fraction to be treated in one pass to obtain a hydrotreated product.
16. The process according to any one of claims 13 to 15, characterized in that the treatment with hydrogen is carried out at a temperature between 100 and 550 ° C, preferably at a temperature between 250 and 450 ° C.
17. The method of claim 16, wherein the hydrogen treatment is carried out at a pressure between 10 and 200 atmospheres.
18. The process of claim 1, wherein hydrogen is separated from the hydrotreated feed and from the hydrotreated product, unless the latter is recovered prior to the hydrogen production stage.
print version
Date of publication 02.03.2007гг




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.