| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2268324
![]()
Electrode for use in hydrogen (Options) and method of manufacturing the (OPTIONS)
Name of the inventor: Howden Hiroёsi (JP); PLA Yasuhide (JP); Saco Kentaro (JP)
The name of the patentee: by Asahi Kasei Kabushiki Kaisha (JP)
Address for correspondence: 129010, Moscow, ul. Boris Spassky, 25, p.3, Ltd. "Gorodissky and Partners", pat.pov. Yu.D.Kuznetsovu, Identification No 595
Starting date of the patent: 2003.03.20
The present invention relates to an electrode for use in electrolysis, in particular to an electrode for use in hydrogen generation, which is used in the electrolysis of sodium chloride based on an ion exchange membrane method, and which is characterized by a low overvoltage over a long period of time. The electrode comprises a conductive substrate and formed thereon a coating layer of a composition obtained by thermal decomposition of the organic acid in the presence of a mixture comprising at least one platinum group metal. Technical effect - to the electrode with stable performance, low overvoltage and a long service life.
DESCRIPTION OF THE INVENTION
The present invention relates to an electrode for use in electrolysis, in particular to an electrode for use in hydrogen, which is used suitably in the electrolysis of sodium chloride using the ion exchange membrane and which is characterized by a low overvoltage over a long period of time.
During the electrolysis of sodium chloride using the ion exchange membrane is one of the most important problems is to reduce energy consumption. Detailed stress analysis used in the electrolysis of sodium chloride with an ion exchange membrane, shows that, except for theoretically compulsory voltages applied voltage includes a voltage difference caused by the ion-exchange membrane, the anode and cathode overvoltage and the voltage depending on the distance between the anode and cathode in the electrolyzer .
Regarding the overvoltage on the electrodes of said different voltages, in particular as regards the anodic overvoltage, then the so-called insoluble electrode is referred to hereinafter as the DSA (from the English Dimension Stable Anode -. Non-consumable and dimensionally stable anode) coated with an oxide of the platinum layer group overvoltage lowered to below 50 mV or less, so that further improvement can be expected.
On the other hand, as the cathode, the conventionally used materials such as mild steel, stainless steel and nickel, exhibit overpotential ranging from 300 to 400 mV. Accordingly, to reduce the activation overvoltage studied surfaces of such materials.
Examples include highly active cathode, made of oxide by thermal spraying of nickel oxide; Cathodes using metal materials based on Raney nickel; cathodes, which takes advantage of the composite plating of nickel and tin; and cathodes based composite plating of activated charcoal and oxides; all such cathodes have been tried as a cathode for hydrogen production of caustic soda.
However, in order to reduce the electrolysis voltage is necessary to further reduce the overvoltage and offers electrodes respectively, based on different concepts.
In the publication JP-B-3-75635 (EP 129734V) on a conductive metal substrate as a coating layer formed from a heterogeneous mixture consisting of a platinum group metal oxide and nickel oxide is produced and thus a low overpotential cathode.
U.S. Patent No. 4668370 a low overvoltage and longer service life of the coating layer is realized by composite plating noble metal oxide and metallic nickel. In the publications of JP-B-6-33481 and JP-B-6-33492 (U.S. Patent 4,900,419 or EP 298055V) as a material used for the electrode coating complex material composed of platinum and cerium allows increasing resistance to poisoning by iron.
U.S. Patents 5645930 and 5882723 are applied onto a conductive base ruthenium chloride, palladium chloride and ruthenium oxide base thus treated is calcined in air and then by chemical reduction of nickel coated, thus increasing the strength of the coating.
In the publication JP-A-11-140680 is formed on a metal base electrode coating layer consisting essentially of ruthenium oxide and then form on the surface of the resulting low-active and a porous protective layer, thus increasing the life of the electrode.
In the publication JP-A-11-158678, an electrode coating layer is formed, which is provided with the thermal decomposition formed on the metal base covering layer of ruthenium oxide, nickel and rare earth metal capable of absorbing hydrogen and thereby prevent electrolytic oxidation by maintaining the cathode potential hydrogen absorption against reverse current, which is the reason for the completion of electrolysis.
In the publication JP-A-11-229170 is provided by electrodeposition of nickel layer in which ruthenium oxide is dispersed, the surface of this layer is covered with a conductive oxide consisting of titanium oxide, and thus increases resistance to mercury poisoning.
However, even in the examples lifespan of the electrodes is small as described above, so the current situation is actually objective is to further increase the electrode lifetime.
Patent Application WO 01/28714 the inner part of the coating layer becomes porous, and therefore, the surface area increases, so increases in resistance to alkali detectable impurities, and is formed with a low overpotential cathode.
The present invention seeks to overcome the problems described above and aims at providing the cathode with stable quality (performance) and excellent low overvoltage (longer) lifetime by applying thermal decomposition method suitable for mass production.
In accordance with the above purpose, and as a result of research directed to overcoming the problems of the cathode and the authors of the present invention described above in the study received the experimental results described below.
(A) As the active electrode active material for a cathode of ruthenium oxide and effective hydrate thereof.
(B) However, when the potential formation of hydrogen ruthenium oxide hydrate is slowly reduced to ruthenium, which causes a structural change.
(C) When ruthenium chloride is thermally decomposed in a reducing atmosphere of hydrogen or inert gas, it is reduced to metallic ruthenium; metallic ruthenium has a high overvoltage and easily peeled from the substrate, thus leading to insufficient durability.
d) by thermal decomposition when the temperature rises, the carbon atoms in oxalic acid exhibit oxidative action, so that the formation of ruthenium oxide is unlikely to occur even when thermal decomposition is carried out in an oxidizing atmosphere. Furthermore, the material subjected to thermal decomposition in the presence of oxalic acid has a low overvoltage and tends to maintain a stable structure even if the hydrogen generation potential, unlike ruthenium metal generated by thermal decomposition in a reducing atmosphere, and thus can maintain a low overvoltage over a long period of time.
(E) salts of lanthanum, cerium and yttrium themselves have insufficient activity at the hydrogen formation, but their oxides during electrolysis converted from particles of normal shape into needle shape and the needle shape play a role in holding the coating layer comprising a ruthenium oxide or from ruthenium hydrate, and thus effectively preventing the physical exfoliation of the coating layer.
The present inventors have completed the present invention by the discovery made as a result of studies described above, such a method which provides a crystal structure, a stable as a coating layer, even by the thermal decomposition in an oxidizing atmosphere to form reducing hydrogen. This allows to obtain a cathode which has a small number of limitations in its manufacture, low cost, and can maintain a low overvoltage over a long period of time.
In other words, the present invention can be characterized as follows:
(1) An electrode for use in hydrogen generation comprising a conductive substrate and formed thereon a coating layer of a composition obtained by thermal decomposition of the organic acid in the presence of a mixture comprising at least one platinum group metal.
(2) An electrode for use in hydrogen generation comprising a conductive substrate and formed thereon a coating layer of a composition obtained by thermal decomposition in the presence of an organic acid mixture containing at least one platinum group metal and at least one metal salt selected from groups lanthanum, cerium and yttrium.
(3) The electrode according to claim. (2), wherein the amount of said at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium in the range from 1/20 to 1/2 mol based on one mol of metal component in said platinum group compound, and the amount of organic acid is in the range from 1/20 to 2 mol based on one mol of metal component in said platinum group compound.
(4) The electrode according to any one of (1) -. (3), wherein the above described platinum group compound is a ruthenium compound and said organic acid is oxalic acid.
(5) An electrode for use in hydrogen generation comprising a conductive base and a coating layer thereon, said coating layer is a composition obtained by thermal decomposition in an oxygen atmosphere, a mixture comprising a ruthenium compound, oxalic acid in a range from 1/20 to 2 mol, and at least one metal salt selected from the group of lanthanum, cerium and yttrium in the range from 1/20 to 1/2 mol based on one mol of metal component in the ruthenium compound.
6) A method for producing an electrode for use in hydrogen generation, comprising the steps of applying onto a conductive base a mixture comprising at least one platinum group compound and thermally decomposing the applied mixture in the presence of an organic acid to form a coating on the conductive base layer.
(7) An electrode for use in hydrogen produced by the method of claim. (6).
(8) The electrode manufacturing method for use in hydrogen generation, comprising the steps of applying onto a conductive base a mixture comprising at least one platinum group metal and at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium, and thermal decomposing the applied mixture in the presence of an organic acid to form a coating on the conductive base layer.
(9) The method according to claim. (8), wherein the amount of said at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium in the range from 1/20 to 1/2 mol based on one mol of metal component in said platinum group compound, and the amount of organic acid is in the range from 1/20 to 2 mol based on one mol of metal component in said platinum group compound.
(10) The method of claim. (6), (8) and (9), wherein the above described platinum group compound is a ruthenium compound and the organic acid is oxalic acid.
(11) The method of claim. (6), (8) and (9), wherein the thermal decomposition is carried out in an oxygen atmosphere.
(12) The method of claim. (10), wherein the thermal decomposition is carried out in an oxygen atmosphere.
The electrode of the present invention is used as a cathode active in chlor-alkali electrolysis process based on ion exchange. Furthermore, the active cathode of the present invention is particularly suitable for use in chlor-alkali electrolysis in an electrolytic cell with a so-called "zero" clearance based on the use of ion exchange membranes, keeps low overvoltage over a long period of time, has a long life and can prevent deterioration of properties an ion exchange membrane, since operation of the cell at the end of the leaching of the electrode is low.
BRIEF DESCRIPTION OF DRAWINGS
 |
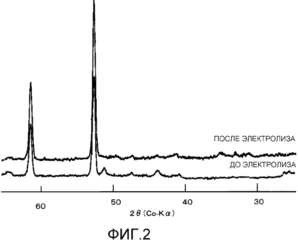 |
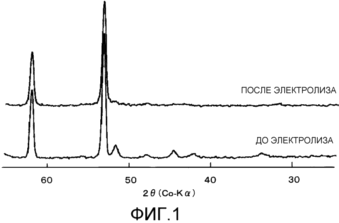
Figure 1 shows the obtained before and after electrolysis for the X-ray diffraction of the coating layer composed of products of thermal decomposition of ruthenium chloride and oxalic acid according to Example 1;
Figure 2 shows the obtained before and after electrolysis for the X-ray diffraction of the coating layer composed of products of thermal decomposition of RuCl 3 + CeCl 3 + oxalic acid according to Example 3;
 |
 |
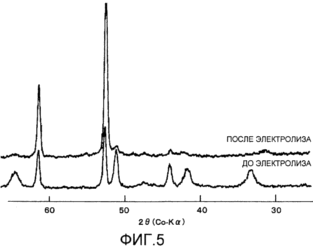 |
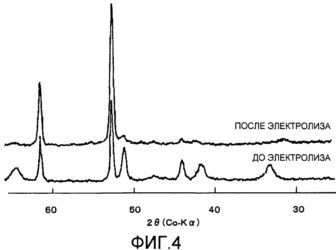
Figure 3 shows the obtained transmission electron micrograph of the layer of the cathode coating after start of the electrolysis (energization) according to Example 3; 4 shows before and after electrolysis X-ray diffraction of the coating layer composed of the pyrolysis product RuCl 3 according to Comparative Example 1; and 5 shows the obtained before and after electrolysis for the coating layer X-ray diffraction patterns consisting of a product of thermal decomposition of RuCl 3 + CeCl 3 according to Comparative Example 2. |
PREFERRED EMBODIMENTS OF THE INVENTION
Since the conductive base is used in an aqueous solution with a high concentration of alkali, it may be made of stainless steel, but of an electrode made of stainless steel, iron and chromium are washed, and the conductivity of stainless steel is about 1/10 the conductivity of nickel, thus advantageously nickel is used.
type bases is not limited in any way, and therefore, according to the intended purpose can be selected by the corresponding form, and are preferably used as the porous base plate, "extended" basis (i.e., a substrate with increased surface area) and a base in the form of a woven mesh, formed by weaving nickel wire. With respect to the form bases should be noted that the appropriate type depends on the distance between the anode and cathode when distance is finite (limited), a base is used as a porous plate or an expanded base; At the same time, when the membrane and the electrodes contact each other, i.e. used zero-gap electrolyzer, a woven mesh is used, made by weaving fine wire or the like
Preferably, such bases were annealed in an oxidizing atmosphere to remove the residual stress, because the residual stress generated during manufacture, is still in it. Also, to form on the surface of the base layer adhesive coating method on the substrate surface using a steel mesh or preferably aluminum oxide powder form irregularities, after which the surface area is increased by acid treatment (etching).
The degree of surface roughness does not specified, but it is desirable to use surface roughness Ra on JIS (Japanese Industrial Standard), preferably of from 1 to 10 micrometers, because the foundation is sometimes used so that it contacts with the ion exchange membrane. Accordingly, it is preferable to sand blasting was done using a mean particle size of 100 micron alumina powder or less, or acid treatment is conducted. As the mineral acid is preferably used (inorganic) acids, including hydrochloric acid, nitric acid, sulfuric acid and phosphoric acid, among which sulfuric acid is preferred from the viewpoint of easier handling. Preferably the acid treatment is conducted at a temperature ranging from 60 to 90 ° C, an aqueous solution of sulfuric acid at a concentration of 10 to 50 wt.%, For 1 to 8 hours.
Preferably, the pretreatment applied to the bases of its aqueous solution containing from 0.001 to 1% surfactant, drying it, and then feed it to a coating solution as described below. Pretreatment of the substrate surface improves the wettability and surface roughness basis so that the coating solution can be deposited uniformly even on the inner parts of the irregularities; respectively, during annealing in the air electrode active material is formed even on the inner parts of the irregularities in the substrate surface. This obviously provides the effect of increasing the surface area and the effect of improving the adhesion between the electrode active material, that is, the electrode coating layer, and the base electrode.
The surfactant is used for the pretreatment described above can be a surfactant of any type, including anionic, cationic and non-ionic type is preferably used with a nonionic surfactant. The amount of surfactant may be small, and is preferably used 0.1-0.01% aqueous solution.
Solution used as a component of the coating of the platinum group metal salt is selected from salts of Pt, salts Ir, salts of Ru, Rh salts, Pd and Os salts salts. Most preferred are ruthenium salts. The salt of the platinum group metal used as a component of solution for coating may be any salt of the chloride, sulfate and nitrate. Preferably, the chloride is used from the standpoint of ease of thermal decomposition and availability of the raw salts. The concentration of metal in the platinum group metal salt solution is not particularly limited, but preferably it is in the range from 10 to 200 g / l, more preferably - in the range from 50 to 120 g / l, in terms of coating thickness in one application.
One can apply any of lanthanum salts, yttrium and cerium salts are preferred but salts of metals such as nitrates, sulfates, chlorides, and chlorides are more preferably used from the viewpoint of convenience upon thermal decomposition and availability of the raw salts.
A substance having an effect of creating a reducing atmosphere during the thermal decomposition is a substance containing carbon, such as oxalic acid, formic acid, acetic acid, citric acid and the like, in which oxalic acid is preferably used. Oxalic acid exists in two forms - and unhydrated as the dihydrate from which dihydrate is preferably used because of its greater availability.
The organic acid may be added to a solution of a mixture comprising at least one platinum group metal and at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium, or, instead of adding to the mixture solution, the organic acid can be placed in an oven at during thermal decomposition. However, it is desirable to mix with the said organic acid is at least a platinum group metal salt and at least one metal salt selected from the group of lanthanum, cerium and yttrium. To a solution of the mixture can be added to other solutions. The mixture may partly remain in solution as a precipitate. Such solutions include water, various alcohols, including propyl alcohol, butyl alcohol, allyl alcohol, or other solvents; Only those solvents should be selected which can be dissolved or suspended mixture. Most preferably, the solution is either an aqueous solution or suspension in water.
With regard to a mixture comprising an organic acid, at least one platinum group metal and at least one metal salt selected from the group of lanthanum, cerium and yttrium, it is preferable that the amount of organic acid is in the range from 1/20 to 2 mol, and the amount of cerium is in the range from 1/20 to 1/2 mol based on one mol of metal component in the platinum group metal salts to the components of the mixture to be thermally decomposed to form a coating layer, creating a sufficient effect.
When the amount of organic acid is less than 1/20 mole per mole of metal component in the platinum group metal salt, the effect of the presence of an organic acid, comprising preventing recovery in the coating layer, it is not sufficiently exerted, while when the amount is larger, than 2 moles, during the preparation of the coating solution produced precipitation etc. The amount of organic acid is preferably in the range of 1/10 to 1 mol and the amount of cerium is in the range from 8.1 to 1.4 mole based on one mole of ruthenium.
As a method of applying onto a conductive base a mixture comprising an organic acid, at least one platinum group metal and at least one metal salt selected from lanthanum group, cerium, and yttrium is preferably used a method of immersing in which the substrate is immersed in a solution coating; brushing method in which the base is treated with a brush, which is dipped in the coating solution; roll coating method in which coating is applied using a sponge roller impregnated with the coating solution; electrostatic coating method in which the coating solution is sprayed so that the coating solution and the base are charged with opposite charges.
Among these methods are preferably used a roller coating method and an electrostatic method, since both of these methods have a high productivity and allow to apply the coating solution evenly.
The coating solution is applied to a substrate, then the base was dried at a temperature ranging from 10 to 50 ° C, and the thermal decomposition is carried out by placing the base in a muffle furnace heated to 300-650 ° C. Thermal decomposition refers to a process in which the mixture containing the starting materials (precursors) are heated to accelerate the decomposition; in this case the pyrolysis means a reaction in which the metal salt is decomposed to the metal and gaseous substances. In particular, the thermal decomposition reaction is as follows: if the metal salt is chloride, the salt is decomposed into chlorine gas and the metal; if the metal salt is a salt of nitric acid, the salt is decomposed into the metal, nitrogen, and gases NO x; and if the metal salt is a salt of sulfuric acid, the salt is decomposed to metal sulfur gases and SO x. On the one hand, for metals should be noted that their reactions depend on the reaction atmosphere, and many metals tend to combine with oxygen and thereby form oxides in an oxygen atmosphere. Oxygen atmosphere means an atmosphere that contains oxygen, but in terms of production cost of oxygen most preferred atmosphere is air.
To accelerate the thermal decomposition of a mixture containing an organic acid, at least one platinum group metal and at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium, it is preferable that the temperature of the thermal decomposition is in the range from 450 to 600 ° FROM. At temperatures below 450 ° C the rate of thermal decomposition of the mixture is low, while at temperatures above 600 ° C dramatically enhanced softening of the nickel base. Accordingly, from the viewpoint of accelerating thermal decomposition of a mixture and maintaining the strength of nickel base, most preferably the temperature range from 500 to 550 ° C. Preferably, in order to achieve the full extent of thermal decomposition duration of the thermal decomposition it was great, but from the standpoint of preventing complete oxidation of the thermally decomposed products and the performance of the electrode manufacturing process, the duration of thermal expansion for one run of thermal expansion is preferably in the range of from 5 to 60 minutes, more preferably - 10 to 30 minutes.
The thermal decomposition is formed on the conductive base coat layer. Predetermined thickness of the coating layer produced according to need by repeating the deposition cycle, drying and annealing for the purpose of thermal decomposition. The thicker the coating layer is, the longer the period during which it is possible to maintain a low overvoltage; however, from the viewpoint of economic efficiency, preferably, the thickness of the coating layer is from 1 to 5 microns. Coating weight and coating amount is preferably from 6 g to 30 g per 1 m 2 of the apparent surface area, more preferably - from 2 to 3 microns, i.e. 12 to 18 g per 1 m 2 of the apparent surface area.
In order to achieve a predetermined thickness can increase the amount of the coating in one application, or it is possible to increase the concentration of the platinum group metal salt; however, when a large amount of the coating during application of the coating can be formed and the roughness may be formed so uneven coating. Therefore, preferably carried out several cycles of application - drying - annealing with the purpose of thermal decomposition. The thickness of the coating layer is achieved in one cycle is preferably controlled in the range of 0.1 to 0.7 microns, more preferably - 0.2 to 0.4 microns.
Preferably, in order to stabilize the coating layer and annealing was carried out after coating a layer of a given thickness, and thus for a long time, so that thermal decomposition of the coating layer may completely pass. The conditions for such annealing temperature range determined from 500 to 650 ° C, preferably - from 500 to 550 ° C. When a short duration of thermal decomposition of the coating layer, this process does not pass the full extent, while when too large duration recovery effect disappears from the presence of an organic acid and, accordingly, an undesirable oxidation of the coating layer. Thus, thermal decomposition of acceptable duration is in the range from 30 minutes to 8 hours, and preferably - it is in the range from one hour to 3 hours.
As the coating layer on the conductive base is provided a composition which is obtained by thermal decomposition in the presence of an organic acid mixture containing at least one platinum group metal (preferably - a ruthenium compound) and at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium.
The action of organic acids (preferably - oxalic acid) is to increase the service life by providing a small structural changes in the structure even in the case where the organic acid is used in a reducing atmosphere of hydrogen due to the formation of such oxides, which have a high degree of crystallinity and is easily restored to hydrogen reducing atmosphere, so that there is a tendency to only minor structural changes. Changes in the layer of the coating observed before and after electrolysis and measured by X-ray diffraction are small. Even when annealing in an atmosphere containing a large amount of oxygen, the formation of oxides which are easily reduced in a hydrogen atmosphere, in fact formed during the electrolysis is negligible. Consequently, we can assume that is formed on the conductive base composition, which is clearly different from the composition prepared by thermal decomposition in the absence of organic acid.
The metal salt selected from lanthanum group cerium and yttrium (preferably - Salt cerium) itself has a low activity for hydrogen production, but oxide obtained from such a salt, is converted in an environment in which hydrogen is produced from particles of normal shape acicular particles in the form and the needles formed play an important role in keeping the coating layer made of a platinum group metal salt, and have an effect of suppressing the physical exfoliation of the coating layer.
The coating layer is structurally stable even in a reducing atmosphere in which hydrogen is generated because the layer has the effect of suppressing the formation of oxides of the platinum group and form the conversion effect poorly active compounds such as lanthanum oxide, cerium oxide and yttrium oxide during the electrolysis process. Thus, it is possible to suppress the physical exfoliation of the coating layer and maintain a low overvoltage over a long period of time. Participation of organic acid during the thermal decomposition does conduct annealing in air possible, which is useful from an industrial point of view.
DETERMINATION ruthenium oxide in the coating layer
In that case, when the chosen ruthenium oxide, ruthenium oxide in the coating layer was determined as follows: a platinum group metal oxide.
A sample of the coating layer formed on the nickel base, placed in a sample holder of an X-diffraction and measurement was carried out using a Co X-ray tube anode X-ray tube or a Cu-anode. Then, comparing the intensity of the most intense peaks of ruthenium oxide and nickel. In particular, the peak area was obtained as the peak height multiplied by its full width at half height, and comparing the thus obtained intensity. Here, the full width at half maximum means the width of the diffraction line at 50% of maximum peak height intensity. When using a Co X-ray tube anode nickel most intense line is at approximately 2 ![]() = 52 °, the most intense line and ruthenium oxide is approximately 2
= 52 °, the most intense line and ruthenium oxide is approximately 2 ![]() = 32,6 °, where
= 32,6 °, where ![]() denotes the diffraction angle.
denotes the diffraction angle.
The intensity ratio between ruthenium oxide and nickel bases preferably 5/100 or less, more preferably - 1/100 or less. When the intensity ratio between ruthenium oxide and nickel bases greater than 5/100, the content of ruthenium oxide increases, and accordingly the reduction of ruthenium oxide extending in a reductive atmosphere where hydrogen is generated vigorously, causing a structural change, leading to flaking of the coating layer. The cause delamination of the coating layer is not clear, but can be attributed to structural change, the caller probably change in crystal structure and the formation of strain in the crystals.
Actually peeling of the coating layer on the cathode with a large content of ruthenium oxide was confirmed when after energization (start of the electrolysis) was conducted with observation by an electron microscope.
MEASUREMENT ELECTRODE SURGE
The overvoltage on the cathode formed thereon with a coating layer for use in hydrogen generation measured by the following method.
The cathode piece cut off size of 48 × 58 mm and drilled two holes in it in order to strengthen this portion in a small cell with screws made of nickel. The electrode is formed by coating on an extended basis, can be evaluated as such; piece of woven mesh, made of thin wire, is fixed on an extended basis, on which no coating film thereon by use of thin nickel wire or the like method and then subjected to measurement. The reference electrode can be a string of platinum wire coated with PFA (copolymer of tetrafluoroethylene and perfluoroalkyl vinyl ether), with a length of exposed platinum portion of about 1 mm, mounted on the electrode surface facing the ion exchange membrane.
As used anode is a so-called anode DSA, consisting of ruthenium oxide, iridium oxide and titanium oxide formed on a titanium-based and rubber gaskets of EPDM (rubber based on ethylene, propylene and a diene monomer) is used as the anode cell and the cathode cell between which is sandwiched a plate exchange membrane for electrolysis. Type ion exchange membrane is in no way limited, but is preferably carried out electrolysis using a cation exchange membrane "Aciplex®", used for the electrolysis of sodium chloride and manufactured by Asahi Kasei Corp.
During electrolysis as the rectifier for electrolysis current pulse generator, a current is passed at a predetermined current density, the current was instantaneously turned off when the analyzing recorder or the like is observed form the resultant wave, and thus the overvoltage was obtained by subtracting the solution resistance between the electrode and the reference electrode.
The electrolysis conditions were as follows: the current density: 3 kA / m 2 or 4 kA / m 2; concentration brine (salt solution) in the anode chamber: 205 g / l; . Alkali concentration in the cathode space 32 wt%; and the electrolysis temperature: 90 ° C. In order to confirm long-term stability was measured electrolysis cathode overvoltages after 30 days from the beginning of electrolysis. Changing the mass of the coating layer was determined as follows: after the electrolysis has ended, loosened screws fixing the electrode to unfasten electrode; detach the electrode is completely washed with water, dried and weighed; so we are comparing the weight before and after electrolysis.
In the following Examples will be given a more detailed description, but the present invention is not limited to these examples.
example 1
Nickel expanded base, in the form of an electrode, the size of the openings towards which the short (SW) was 3 mm, the size of the holes along the long direction (LW) was 4.5 mm, perforation "expansion" processing was 0.7 mm, and the thickness of the plate was 0.7 mm, was calcined in air at 400 ° C for 3 hours thereby forming on the surface of the oxide coating layer. Subsequently, blasting powder with an average particle size of 100 micron or less aluminum oxide to provide the base surface with irregularities. Then, the base was subjected to acid treatment in a sulfuric acid concentration of 25 wt.% At 90 ° C for 4 hours and provided on the substrate surface with finer irregularities.
Then, the nickel base was soaked into a solution in water with dissolved surfactant "Nonion N210" (trade mark: a nonionic surface active agent company NOF Corp. (Nippon Yushi KK)) of 0.15 g surfactant per 200 g water, taken out and then the base air-dried.
Then, ruthenium chloride solution in 6% hydrochloric acid with a metal concentration of 100 g / l of oxalic acid dihydrate was added so that the molar amount of oxalic acid dihydrate was 0.5 times the molar amount of ruthenium, and the mixture thus obtained was stirred at 90 ° C for one day to yield a mixture composed of ruthenium chloride and oxalic acid.
Then the mixture was immersed in a nickel base, foundation dried at 50 ° C for 10 minutes and calcined at 500 ° C for 10 minutes in air. Then, the combined process of dipping in a solution containing oxalic acid and ruthenium, drying and calcination at 500 ° C was repeated a total of 5 times and then the base was calcined at 550 ° C for one hour.
The cathode was in this condition was cut into a piece of 48 × 58 mm, the piece was fixed in a small cell and subjected to the overvoltage evaluation. For the purpose of making the piece removable severed portion of the cathode of 48 × 58 mm was fixed to the rib of the nickel cell body with nickel screws. As the reference electrode, platinum wire, fixed longitudinally from a PFA coated platinum bared over a length of about 1 mm. The applied anode used was a so-called anode DSA, consisting of ruthenium oxide, iridium oxide and titanium oxide formed on a titanium base, the anode cell and the cathode cell used were the rubber gasket of EPDM (rubber based on ethylene, propylene and diene monomer), and the ion exchange membrane is a "Aciplex®" F4203 firm Asahi Kasei Corp.
During electrolysis as the rectifier for electrolysis current pulse generator "114 NA ™" firm Hokuto Denko Co., Ltd. The electrolysis conditions were as follows: the current density: 4 kA / m 2; concentration brine (salt solution) in the anode chamber: 205 g / l; . Alkali concentration in the cathode chamber: 32 wt%; and the electrolysis temperature: 90 ° C. After 3 days and 30 days from the beginning of electrolysis, the cathode overvoltage was measured. The cathode overvoltages were derived as follows.
The cathode voltage E 1 relative to a platinum wire at the current density of 4 kA / m 2, then the voltage E2 was measured at the time when the current pulse generator means "NS114 ™" firm Hokuto Denko Co., Ltd current was instantaneously turned off. E 2 represents the measured voltage associated with the structural resistance and solution resistance. Thereby, as the overvoltage E 1 -E 2.
Change of mass was prepared as follows: after the electrolysis had been terminated, loosened screws fixing the electrode to unfasten electrode unfixed electrode was fully washed with water, dried and weighed; mass compared before and after electrolysis.
Thus we found that after 3 days, the overvoltage was 74 mV and the weight change was 0 mg. The same cathode was subjected to the subsequent evaluation of the electrode characteristics, and found that even after 30 days, the overvoltage was 73 mV and the weight change was 1 mg, which proves that the obtained cathode having a low overvoltage and a very long lifetime.
Furthermore, on a diffractometer Rigaku-Denki Geiger flex 4036A2 X-ray from the X-ray tube with Co-removed anode X-ray diffraction of the sample cathode. The results are shown in Figure 1.
Before energization (electrolysis) of the most intense peak of the nickel base was found around 52 °, and no peaks of ruthenium oxide were found around 32 °, 42 ° and 65 °. After energization (electrolysis) changes were scarcely found except for the advent of the peaks at about 44 ° and 51 °, due to nickel oxide, and the electron micrograph taken after energization, peeling of the coating layer was found.
examples 2-6
Nickel expanded base, in the form of an electrode, the size of the openings towards which the short (SW) was 3 mm, the size of the holes along the long direction (LW) was 4.5 mm, perforation "expansion" processing was 0.7 mm, and the thickness of the plate was 0.7 mm, was calcined in air at 400 ° C for 3 hours thereby forming on the surface of the oxide coating layer. Subsequently, blasting powder with an average particle size of 100 micron or less aluminum oxide to provide the base surface with irregularities. Then, the base was subjected to acid treatment in a sulfuric acid concentration of 25 wt.% At 90 ° C for 4 hours and provided on the substrate surface with finer irregularities.
Then, the nickel base was soaked into a solution in water with dissolved surfactant "Nonion N210" (trade mark: a nonionic surface active agent company NOF Corp. (Nippon Yushi KK)) of 0.15 g surfactant per 200 g water, taken out and then the base air-dried.
Subsequently, to a solution of ruthenium chloride at a concentration of metal of 100 g / l was added oxalic acid dihydrate, such that the molar ratio of oxalic acid dihydrate, based on one mole of ruthenium corresponds to the values shown in Table 1 was further added cerium chloride so that the molar ratio cerium, based on one mole of ruthenium corresponds to the values shown in table 1, and the resulting mixture was stirred at 90 ° C for one day to yield a mixture composed of ruthenium chloride, cerium chloride and oxalic acid.
Then the mixture was immersed in a nickel base, foundation dried at 50 ° C for 10 minutes and calcined in air at 500 ° C for 10 minutes. Then, the total immersion process into a mixture, drying and calcination at 500 ° C was repeated for a total of 10 times, and finally the base was calcined at 550 ° C for one hour. The thickness of the coating layer after anneal ranged from 2 to 3 microns.
The cathode was in this condition was cut into a piece of 48 × 58 mm, the piece was fixed in a small cell and subjected to the overvoltage evaluation. The cathode piece of 48 × 58 mm with the purpose of making the piece removable, was fixed to the rib of the nickel cell body with nickel screws. As the reference electrode of platinum wire coated with PFA exposed platinum portion was about 1 mm long reinforced longitudinally on a surface contacting the ion exchange membrane. The applied anode used was a so-called anode DSA, consisting of ruthenium oxide, iridium oxide and titanium oxide formed on a titanium base, the anode cell and the cathode cell used were the rubber gasket of EPDM (rubber based on ethylene, propylene and diene monomer), and the ion exchange membrane is a "Aciplex®" F4203 firm Asahi Kasei Corp.
During electrolysis as the rectifier for electrolysis current pulse generator "NS114 ™" firm Hokuto Denko Co., Ltd. The electrolysis conditions were as follows: the current density: 3 kA / m; concentration brine (salt solution) in the anode chamber: 205 g / l; . Alkali concentration in the cathode chamber: 32 wt%; and the electrolysis temperature: 90 ° C. 30 days after the beginning of electrolysis, the cathode overvoltage was measured.
The cathode overvoltages were derived as follows. The cathode voltage E 1 relative to the reference electrode at a current density of 3 kA / m 2, then the voltage E2 was measured at the time when the current pulse generator means "NS114 ™" current was instantaneously turned off. E2 corresponded to the voltage associated with the structural resistance and solution resistance. Thus, the net overvoltage was derived as E 1 -E 2.
Changing the weight of the coating layer was prepared as follows: after the electrolysis had been terminated, loosened screws fixing the electrode to unfasten electrode unfixed electrode was fully washed with water, dried and weighed; thus, from the weights before electrolysis and after 30 days of energization obtained mass change. The results are shown in Table 1.
| Table 1 | |||||
| Example | 2 | 3 | 4 | 5 | 6 |
| Oxalic acid mol | 1 | 1/2 | 1/4 | 1/2 | 1/10 |
| Number of Ce mole | 1/4 | 1/4 | 1/4 | 1/8 | 1/4 |
| Overvoltage, mV | 72 | 69 | 73 | 74 | 71 |
| Reducing the coating weight, mg | 3 | 2 | 3 | 4 | 2 |
In these examples, shown in Table 1, the electrodes prepared with high durability, which are characterized by low overvoltage and a small reduction in weight of the electrode coating layer.
Diffractogram cathode sample prepared by applying a mixture of 1 mol of ruthenium - 2.1 mol of oxalic acid - 4.1 mole Ce of Example 3, were recorded on a diffractometer Rigaku-Denki Geiger flex 4036A2 X-ray from a Co X-ray tube anode. The results are shown in Figure 2.
Before energization, the most intense peak of the nickel base was found around 52 °, and no peak of ruthenium oxide was detected at about 32 °. After energization, changes were scarcely found except for the advent of the peaks at about 44 ° and 51 °, due to nickel oxide, and the electron micrograph taken after energization, peeling of the coating layer was found.
Cathode coating layer peeled from a sample of porous nickel base after 30 days of energization, the cross section of the sample was treated to a specified condition and then observation performed with a transmission electron microscope. The resulting electron micrograph is shown in Figure 3. The results confirm the effect of turning the observation cerium needle-shaped particles in the areas (1) and (2) in Figure 3, with such needle-like particles play an important role in holding the coating layer (3) consisting of ruthenium oxide and ruthenium hydrate, thus that the peeling of the coating layer is suppressed.
The void between the detected portions (1) and (3) in Figure 3, was formed during the preparation of the sample for observation with a transmission electron microscope to observe the state of allowing clear needle-like particles.
example 7
The basis of a woven mesh, made of thin nickel wire 0.15 mm in diameter with an equal number of holes 50 per linear inch, calcined in air at 400 ° C for 3 hours to form a layer of an oxide film on its surface. Subsequently, blasting powder with an average particle size of 100 micron or less aluminum oxide to provide the base surface with irregularities. Then, the base was subjected to acid treatment in a sulfuric acid concentration of 25 wt.% At 90 ° C for 4 hours and provided on the substrate surface with finer irregularities.
Then, the nickel base was soaked into a solution in water with dissolved surfactant "Nonion N210" (trade mark: a nonionic surface active agent company NOF Corp. (Nippon Yushi KK)) of 0.15 g surfactant per 200 g water, taken out and then the base air-dried.
Then a solution of ruthenium chloride in hydrochloric acid with a metal concentration of 100 g / l of oxalic acid dihydrate was added so that the amount of oxalic acid dihydrate was 0.5 mole based on one mole of ruthenium, then added to a solution of cerium chloride so that the amount of ceria is 0.5 mole based on one mole of ruthenium, and the mixture thus obtained was stirred at 90 ° C for one day to yield a mixture composed of ruthenium chloride, cerium chloride and oxalic acid.
Applying the solution to a woven mesh for forming the coating was carried out using a device for roller coating, in which the bath with the coating solution was placed in the lowest position of the device, roller coating, made of EPDM, was impregnated with the coating solution, the other roll was placed above of the EPDM roller so that there is always contact with the roller of EPDM, and further roller arranged above the PVC roller mounted above the roller of EPDM.
Surfaces to which the applied coating solution, held between a pair of sponge rollers made of EPDM, directly before the base was dried, a coating solution that accumulates at the intersections of the woven mesh was captured and removed. Then, the base of the metal mesh was dried at 50 ° C for 10 minutes, calcined in air at 500 ° C for 10 minutes, a combination of operations roll coating, drying and calcination at 500 ° C was repeated for a total of 10 times, and then was annealing at 550 ° C for one hour.
The cathode was in this condition was cut into a piece of 48 × 58 mm, the piece was fixed in a small cell and subjected to the overvoltage evaluation. Cathode piece cut into the size of 48 × 58 mm with a view to making the piece removable fastened on an extended basis, having no coating film thereon by use of thin nickel wire or the like manner, and then the base was fixed to the rib of the nickel cell body with nickel screws. As a reference electrode, a platinum wire coated with PFA of the exposed platinum portion was about 1 mm in length, fixed longitudinally on a surface contacting the ion exchange membrane. The applied anode used was a so-called anode DSA, consisting of ruthenium oxide, iridium oxide and titanium oxide formed on a titanium base, the anode cell and the cathode cell used were the rubber gasket of EPDM (rubber based on ethylene, propylene and diene monomer), and the ion exchange membrane is a "Aciplex®" F4203 firm Asahi Kasei Corp.
During electrolysis as the rectifier for electrolysis current pulse generator "NS114 ™" firm Hokuto Denko Co., Ltd. The electrolysis conditions were as follows: the current density: 3 kA / m 2; concentration brine (salt solution) in the anode chamber: 205 g / l; . Alkali concentration in the cathode chamber: 32 wt%; and the electrolysis temperature: 90 ° C. 30 days after the beginning of electrolysis, the cathode overvoltage was measured.
The cathode overvoltages were derived as follows. The cathode voltage E 1 relative to the reference electrode at a current density of 3 kA / m 2, then the voltage E2 was measured at the time when the current pulse generator means "NS114 ™" firm Hokuto Denko Co., Ltd current is instantaneously turned off. E2 corresponded to the voltage associated with the structural resistance and solution resistance. Thus, the net overvoltage was derived as E 1 -E 2.
Reducing the weight of the coating layer was prepared as follows: after the electrolysis had been terminated, loosened screws fixing the electrode to unfasten electrode unfixed electrode was fully washed with water, dried and weighed; weight reduction was obtained from the weights before electrolysis and after 30 days of energization.
30 days after the start of the electrolysis, the overvoltage was 68 mV, and reduction in weight of the coating was 2 mg, and thereby a cathode having a low overvoltage and long lifetime.
Comparative Example 1
The cathode was produced on the basis of the same operations as in Example 1 except that the used ruthenium chloride solution in 6% hydrochloric acid with a metal concentration of 100 g / l.
In other words, an extended base in the form of nickel electrode in which the size of the openings towards the short (SW) was 3 mm, the size of the holes along the long direction (LW) was 4.5 mm, perforation "expansion" processing was 0.7 mm and the plate thickness was 0.7 mm, annealed in air at 400 ° C for 3 hours and thus formed on the surface of the oxide coating layer. Subsequently, blasting powder with an average particle size of 100 micron or less aluminum oxide to provide the base surface with irregularities. Then, the base was subjected to acid treatment in a sulfuric acid concentration of 25 wt.% At 90 ° C for 4 hours and provided on the substrate surface with finer irregularities.
Then, the nickel base was soaked into a solution in water with dissolved surfactant "Nonion N210" (trade mark: a nonionic surfactant company NOF Corp. (Nippon Yushi K.K.)) of 0.15 g of surfactant 200 g of water, then the base fetched and air dried.
Then, the nickel base was soaked into the ruthenium chloride solution in 6% hydrochloric acid with a metal concentration of 100 g / l and dried at 50 ° C for 10 minutes, and then annealed in air at 500 ° C for 10 minutes. Then the soaking into the ruthenium chloride solution, drying and calcination at 500 ° C was repeated a total of 5 times, and then calcination was conducted at 550 ° C for one hour.
The cathode was in this condition was cut into pieces of 48 × 58 mm, the piece was fixed in a small cell and subjected to the overvoltage evaluation. Cathode piece cut into the size of 48 × 58 mm with the purpose of making the piece removable, was fixed to the rib of the nickel cell body with nickel screws.
As a reference electrode, a platinum wire coated with PFA of the exposed platinum portion was about 1 mm in length, fixed longitudinally. The applied anode used was a so-called anode DSA, consisting of ruthenium oxide, iridium oxide and titanium oxide formed on a titanium base, the anode cell and the cathode cell used were the rubber gasket of EPDM (rubber based on ethylene, propylene and diene monomer), and the ion exchange membrane is a "Aciplex®" F4203 firm Asahi Kasei Corp.
During electrolysis as the rectifier for electrolysis current pulse generator "NS114 ™" firm Hokuto Denko Co., Ltd. The electrolysis conditions were as follows: the current density: 4 kA / m 2; концентрация рассола (раствора соли) в анодной камере: 205 г/л; концентрация щелочи в катодной камере: 32 мас.%; и температура электролиза: 90°С. Спустя 3 дня после начала электролиза измеряли катодное перенапряжение.
Катодное перенапряжение получали следующим образом.
Измеряли катодное напряжение Е 1 относительно платиновой проволоки при плотности тока 4 кА/м 2 . Напряжение E 1 включает в себя катодное перенапряжение, сопротивление раствора между электродом сравнения и катодом, сопротивление структуры никелевой ячейки и сопротивление контактов между электродом и фланцем. Затем измеряли напряжение Е 2 в тот момент времени, когда посредством импульсного генератора тока "НС114™" ток мгновенно выключается. Когда ток мгновенно выключается, катодное перенапряжение мгновенно понижается, и напряжение Е 2 становится напряжением, связанным с описанным выше сопротивлением раствора, сопротивлением структуры и сопротивлением на контактах, и таким образом перенапряжение в контуре получают как E 1 -Е 2 .
Изменение массы до и после электролиза получали следующим образом: после того, как электролиз заканчивали, ослабляли винты, закрепляющие электрод, чтобы открепить электрод, незакрепленный электрод полностью промывали водой, сушили и взвешивали; таким образом, сравнивали массы до и после электролиза.
Из полученных результатов находили перенапряжение, равное 75 мВ, и уменьшение массы, равное 20 мг. Тот же самый катод применяли в последующем электролизе, и спустя 30 дней найденное перенапряжение составляло 82 мВ, а дополнительное уменьшение массы составляло 22 мг.
Дифрактограммы, полученные с помощью рентгеновской трубки с Со-анодом, приведены на фиг.4. До включения напряжения наиболее интенсивный пик никелевой основы обнаруживали приблизительно при 52°, пик оксида рутения обнаруживали приблизительно при 32°, и другие пики оксида рутения обнаруживали приблизительно при 42° и 65°; рассчитанное отношение интенсивности пиков составляло 50/100, указывая, что содержание оксида рутения в данном примере является большим. После включения напряжения пики, отличающиеся от пиков никелевой основы приблизительно при 61° и 52°, практически исчезли.
Наблюдение образца после электролиза в электронном микроскопе привело к подтверждению того явления, что слой электродного покрытия физически отслаивается от поверхности основы. Отслаивание от поверхности электрода, вероятно, привело к уменьшению массы слоя покрытия.
Сравнительный пример 2
The cathode was obtained by the same operations as in Examples 2-7 except that the used aqueous solution of ruthenium chloride at a concentration of 100 g / liter and an aqueous solution of cerium chloride.
In other words, the nickel base, wherein the size of holes in the short direction (SW) was 3 mm, the size of the holes along the long direction (LW) was 4.5 mm, perforation "expansion" processing was 0.7 mm, and the thickness of the plate It was 0.7 mm, annealed in air at 400 ° C for 3 hours and thus formed on the surface of the oxide coating layer. Subsequently, blasting powder with an average particle size of 100 micron or less aluminum oxide to provide the base surface with irregularities. Then, the base was subjected to acid treatment in a sulfuric acid concentration of 25 wt.% At 90 ° C for 4 hours, and thus provided with finer irregularities on the substrate surface.
Then, the nickel base was soaked into a solution in water with dissolved surfactant "Nonion N210" (trade mark: a nonionic surfactant manufactured by NOF Corp. (Nippon Yushi KK)) of 0.15 g of the surfactant per 200 g of water, then the base fetched and air dried.
Then, to the aqueous solution of ruthenium metal chloride concentration of 100 g / liter of cerium chloride were added so that the content of cerium was 4.1 mole based on one mole of ruthenium; The mixture thus obtained was stirred at 90 ° C for one day, and thus a mixture containing the ruthenium chloride and cerium chloride. Then, the nickel base was soaked into the mixture solution and then dried at 50 ° C for 10 minutes and calcined in air at 500 ° C for 10 minutes. Then the soaking into the mixture, drying and calcination at 500 ° C was repeated for a total of 10 times, and finally the base was calcined at 550 ° C for one hour.
The cathode was in this condition was cut into pieces of 48 × 58 mm, the piece was fixed in a small cell and subjected to the overvoltage evaluation. The cathode piece cut into a piece of 48 × 58 mm, with the purpose of making the piece removable, was fixed to the rib of the nickel cell body with nickel screws.
As a reference electrode, a platinum wire coated with PFA of the exposed platinum portion was about 1 mm in length, fixed longitudinally on a surface contacting the ion exchange membrane. The applied anode used was a so-called anode DSA, consisting of ruthenium oxide, iridium oxide and titanium oxide formed on a titanium base, the anode cell and the cathode cell used were the rubber gasket of EPDM (rubber based on ethylene, propylene and diene monomer), and the ion exchange membrane is a "Aciplex®" F4203 firm Asahi Kasei Corp.
During electrolysis as the rectifier for electrolysis current pulse generator "NS114 ™" firm Hokuto Denko Co., Ltd. The electrolysis conditions were as follows: the current density: 3 kA / m 2; concentration brine (salt solution) in the anode chamber: 205 g / l; . Alkali concentration in the cathode chamber: 32 wt%; and the electrolysis temperature: 90 ° C. 30 days after the beginning of electrolysis, the cathode overvoltage was measured.
Cathode overvoltage was measured as follows. The cathode voltage E 1 relative to the reference electrode at a current density of 3 kA / m 2, then the voltage E2 was measured at the time when the current pulse generator means "NS114 ™" current was instantaneously turned off. E 2 corresponds to the voltage associated with the structural resistance and solution resistance, and therefore, overvoltage was derived as the E 1 -E 2.
Reducing the weight of the coating layer was prepared as follows: after the electrolysis had been terminated, loosened screws fixing the electrode to unfasten electrode unfixed electrode was fully washed with water, dried and its weight was measured; thus, weight reduction obtained with weights before electrolysis and after 30 days after the beginning of electrolysis. It was found that after 30 days after switching over-voltage voltage was 91 mV, and the weight has decreased by 20 mg.
5 shows the X-ray diffraction pattern of the sample of Comparative Example 2 obtained by using a Co X-ray tube anode before and after energization.
According to these diffractograms energization, the most intense peak of the nickel base was found around 52 °, a peak of ruthenium oxide was found around 32 °, and other peaks of ruthenium oxide were found around 42 ° and 65 °; calculated peak intensity ratio was about 95/100, indicating that the ruthenium oxide content in this example is large. It can be seen that after the energization, the peaks other than peak of the nickel base around 61 ° and 52 °, virtually disappeared.
In addition, the observation sample after energization with an electron microscope led to the confirmation of the phenomenon that the electrode coating layer was physically exfoliated from the base surface. The exfoliation from the electrode surface probably reduces the weight of the coating layer.
industrial applicability
The electrode of the present invention is suitable for use in chlor-alkali electrolysis, and in particular, suitable for use in zero gap electrolysis, wherein the membrane and the electrodes contact with each other, whereby the electrode can maintain a low overvoltage over a long period of time.
CLAIM
1. An electrode for use in hydrogen generation comprising a conductive substrate and formed thereon a coating layer of a composition obtained by thermal decomposition in the presence of an organic acid containing at least one platinum group metal.
2. An electrode for use in hydrogen generation comprising a conductive substrate and formed thereon a coating layer of a composition obtained by thermal decomposition in the presence of an organic acid containing at least one platinum group metal and at least one metal salt selected group of lanthanum, cerium and yttrium.
3. An electrode according to claim 2, wherein the amount of said at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium in the range from 1/20 to 1/2 mol based on one mol of metal component in the platinum group metal and the amount of organic acid is in the range from 1/20 to 2 mol based on one mol of metal component in said platinum group compound.
4. An electrode according to any one of claims 1-3, wherein the above described platinum group compound is a ruthenium compound and said organic acid is oxalic acid.
5. An electrode for use in hydrogen generation comprising a conductive base and a coating layer thereon, said coating layer is a composition obtained by thermal decomposition in an oxygen atmosphere, a mixture comprising a ruthenium compound, oxalic acid in a range from 1/20 to 2 mol and at least one metal salt selected from the group of lanthanum, cerium and yttrium in the range from 1/20 to 1/2 mol based on one mol of metal component in the ruthenium compound.
6. A method of manufacturing an electrode for use in hydrogen generation, comprising the steps of applying onto a conductive base a mixture comprising at least one platinum group compound and thermally decomposing the applied mixture in the presence of an organic acid to form a coating on the conductive base layer.
7. An electrode for use in hydrogen generation, obtained by the method according to claim 6.
8. A method of manufacturing an electrode for use in hydrogen generation, comprising the steps of applying onto a conductive base a mixture comprising at least one platinum group metal and at least one metal salt selected from the group of lanthanum, cerium and yttrium and thermally decomposing the applied mixture in the presence of an organic acid to form a coating on the conductive base layer.
9. The method of claim 8, wherein the amount of said at least one metal salt selected from the group consisting of lanthanum, cerium and yttrium in the range from 1/20 to 1/2 mol based on one mol of metal component in the platinum group metal and the amount of organic acid is in the range from 1/20 to 2 mol based on one mol of metal component in said platinum group compound.
10. The method according to any one of claims 6, 8 and 9, wherein the above described platinum group compound is a ruthenium compound and the organic acid is oxalic acid.
11. The method according to any one of claims 6, 8 and 9, wherein the thermal decomposition is carried out in an oxygen atmosphere.
12. The method of claim 10, wherein the pyrolysis is conducted in an oxygen atmosphere.
print version
Publication date 02.03.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.