|
Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2120156
![]()
UNINSTALLED ALLOY FOR ELECTROCHEMICAL HYDROGEN ACCUMULATION AND CELL FOR ELECTROCHEMICAL HYDROGEN ACCUMULATION
The name of the inventor: Stanford R. Ovshinsky (US); Michael A.Fetchenko (US)
The name of the patentee: Owonik Battery Company, Inc. (US)
Address for correspondence:
Date of commencement of the patent: 1993.08.25
The invention relates to alloys for the electrochemical accumulation of hydrogen and batteries based on them. The technical result is an improvement in performance. A disordered alloy for the electrochemical accumulation of hydrogen and an electrochemical cell having a positive electrode, a spacer and a negative electrode containing this alloy, wherein the alloy has a composition (base alloy) a Co b Mn c Al d Fe c La f Mo g , wherein the base alloy is Disordered multicomponent alloy having at least one structure selected from the group consisting of amorphous, microcrystalline, polycrystalline, with no long-range composite order relative to the composition, with three or more phases of said polycrystalline structure, and any combination of these structures, b is up to 7 , 5 atm.%, C - 0.1 - 8.5 atm.%, D - up to 2.5 atm.%, E - 0.1 - 6.5 atm.%, F - up to 4.5 atm. %, G - up to 6.5 atm.%, B + c + d + e + f + g> 0 and a + b + c + d + e + f + g = 100 atm. %.
DESCRIPTION OF THE INVENTION
The invention is a partial continuation of US Patent Application No. 07 / 746,015.
This invention relates to alloys for the electrochemical accumulation of hydrogen and to rechargeable electrochemical cells using these alloys.
More particularly, the invention relates to rechargeable cells and batteries having negative electrodes consisting of multicomponent alloys for the electrochemical accumulation of hydrogen. Cells that include these alloys have performance characteristics such as energy density, charge retention, number of cycles and low temperature characteristics that significantly exceed the known rechargeable cells using hydrogen storage alloys.
Rechargeable cells that use a nickel-hydroxide positive electrode and hydrogen storage negative electrode formed by a metal hydride ("metal hydride cells") are known from the literature.
When the electric potential is applied between the electrolyte and the metal hydride electrode in the metal hydride cell, the material of the negative electrode (M) is charged by electrochemical adsorption of hydrogen and the electrochemical evolution of the hydroxyl ion; When discharged, dissolved hydrogen is liberated, forming water molecules and secreting an electron ![]()
The reactions that take place on the positive electrode of the nickel metal hydride cell are also reversible. Most metal hydride cells use a nickel hydroxide positive electrode. The following charge and discharge reactions take place at the nickel-hydroxide positive electrode ![]()
In a metal hydride cell having a nickel-hydroxide positive electrode and a negative electrode storing hydrogen, the electrodes are usually separated by a napkin or felted material, nylon or polypropylene. The electrolyte is usually an aqueous solution of alkali, for example 20-45% by weight of potassium hydroxide.
The first hydrogen storage alloys that were investigated as materials for the battery electrodes were TiNi and LaNi 5 . Many years have been spent in the studies of these simple binary intermetallic compounds, as they are known to have an appropriate hydrogen bond strength for use in electrochemical applications. Despite numerous attempts, however, as the researchers found, these intermetallic compounds should be extremely unstable and can not have any electrochemical value due to a variety of harmful properties such as slow discharge, oxidation, corrosion, poor kinetics and poor catalysis. These simple alloys for use in batteries reflect the traditional predilection of battery developers to use simple element compounds in crystalline materials such as NiCd, NaS, LiMS, ZnBr, NiFe, NiZn and Pb-acid. In order to improve the electrochemical properties of binary intermetallic compounds, while maintaining the efficiency of hydrogen storage, the first studies began to modify the TiNi and LaNi 5 systems.
The modification of TiNi and LaNi 5 was initiated by Stanford R. Ovshinsky from Energy Conversion Devices (ECD) Trog, Michigan, USA. Ovshinsky and his colleagues at the ECD found that the hope for simple, relatively clean connections was the main drawback of the well-known works. In the previous work, it was determined that the catalytic action depends on surface reactions in areas with a disruption of the crystal structure. Relatively pure compounds are found to have a relatively low density of hydrogen accumulation sites, and the available type of sites occurs randomly and is not created in the bulk of the material. Thus, the efficiency of the accumulation of hydrogen and the subsequent release of hydrogen to form water, as determined, is substantially less than what would be possible if a greater number and variety of active sites were available.
Ovshinsky first discovered that the number of surface areas could be significantly increased by creating an amorphous film that is similar to the surface of the desired relatively pure materials. As Ovshinsky explained and the coding of optical information, 42 Journal De Physique at C4-1096 (Octobre 1981):
Amorphousness is the initial term, referring to the lack of evidence by X-ray diffraction of the long-range order periodicity, and is not a sufficient description of the material. To understand amorphous materials, several important factors must be considered: the type of chemical bonding the number of bonds arising due to the local order, what is their coordination, and the influence of the entire local environment, both chemical and geometric, on the resulting different configurations. Amorphousness is not determined by the random packing of atoms, regarded as solid spheres or an amorphous solid, which alone contains atoms located in disorder. Amorphous materials should be considered as consisting of an interacting matrix whose electronic configurations are formed by free energy forces, and they can be specifically determined by the chemical nature and coordination of the constituent atoms. Using elements with valence electrons on various orbital and different technologies of preparation, one can bypass the usual relaxation processes that reflect the equilibrium conditions, and, thanks to the three-dimensional freedom of the amorphous state, create completely new types of amorphous materials-chemically modified materials ...
Only by understanding amorphism as a means for introducing surface areas into the film is it possible to get a "mess" not only in amorphous materials, but also in crystalline materials; "Disorder", which takes into account the full range of local order effects, such as porosity, topology, crystallites, site characteristics and spacing between sites. Thus, what to look for modifications of materials that would give ordered materials having the maximum number of randomly generated surface irregularities, the Ovshinsky group in the ECD began designing "disordered materials" where the desired irregularities can be created by order. See US Patent No. 4,623,597, the disclosure of which is incorporated by reference.
The term "disordered", as used herein, is used to denote a term, as it is used in the literature, as follows.
An unordered semiconductor can exist in several structural states. This structural factor constitutes a new variable by means of which the physical properties (of the material) ... can be controlled. Moreover, structural disorder opens up the possibility of obtaining new compositions and mixtures in the metastable state, which far exceeds the limits of thermodynamic equilibrium. So, we mark the following as a further outstanding feature. In many disordered materials ... it is possible to control the short-range order parameter and thereby achieve abrupt changes in the physical properties of these materials, including obtaining new coordination numbers for the elements: SR Ovshinsky, Disorder Form, 32 Journal of Non-Crystalline Solids at 22 (1979) (emphasis added).
The "near order" of these disordered materials was further explained by Ovshinsky in "Chemical basis of amorphousness: structure and function", 26: 8 - 9 rev. Roum. Phys. At 893 - 903 (1981):
The short-range order is not preserved ... In fact, when the crystal symmetry is broken it becomes impossible to maintain the same short-range order. The reason for this is that the short-range order is controlled by the force fields of the electronic orbitals, therefore, the environment must be fundamentally different, in the corresponding crystalline and amorphous solids. In other words, there is an interaction of local chemical bonds with their closest environment, which determines the electrical, chemical and physical properties of the material, and they can never be the same in amorphous materials as they are in crystalline materials. . . Orbital relationships, which may be in three-dimensional space in amorphous but not crystalline materials, are the basis for new geometries, many of which are essentially anti-crystalline in nature. The destruction of bonds and the displacement of atoms can be an adequate reason for the occurrence of amorphism in one-component materials. But to understand amorphism sufficiently, it is necessary to understand the three-dimensional relationships existing in the amorphous state, for which they exist, which cause an internal topology incompatible with the translational symmetry of the crystal lattice ... What is important in the amorphous state is the fact that it is possible to create an infinite A lot of materials that do not have any crystal correspondences, and even those that are initially similar in chemical composition. The spatial and energy relationships of these atoms can be completely different in amorphous and crystalline form, even though the chemical elements may be the same ...
The closest or local order is considered in Ovshinsky's US Patent No. 4,5200,393, entitled "Composite Variable Materials and a Synthetic Material Synthesis Method", the contents of which are incorporated by reference. This patent discusses how disordered materials do not require any periodic local order, and how, using Ovshinsky's technology, it is possible for the spatial and orientational arrangement of similar or different atoms or groups of atoms with such increased accuracy and control of local configurations that it is possible to obtain qualitatively new phenomena . In addition, the patent discusses that the atoms used need not be limited to d-shell or f-shell atoms but can be any atom in which controlled aspects of interaction with the local environment play a significant role physically, electrically or chemically, So as to affect the physical properties and, therefore, the function of the materials. These technologies result in the means of synthesizing new materials that are disordered in several different senses simultaneously.
By obtaining metal hydride alloys from such disordered materials, Ovshinsky and his team were able to greatly increase the reversible oxygen storage characteristics required for efficient and economical applications in batteries and produce batteries having a high density of stored energy, effective reversibility, high electrical efficiency , Volumetric dissolution of hydrogen without structural change or poisoning, a large number of cycles and the ability of a deep discharge.
The improved characteristics of these alloys are the result of the construction of a local chemical order, and therefore of a local structural order, by introducing the selected modifying elements into the original matrix. Disordered metal hydride alloys have a substantially increased density of catalytically active sites and accumulation sites compared to simple, ordered crystalline materials. These additional areas are responsible for the increased efficiency of the electrochemical charge / discharge and the increase in the storage capacity of electrical energy. The nature and number of storage areas can even be set independently of the catalytically active sites. More specifically, these alloys are designed to allow bulk dissolution of dissociated hydrogen atoms with bond strengths within the reversibility range suitable for use in repeated applications of batteries.
Based on the pioneering principles described above, some of the most effective materials for the electrochemical accumulation of hydrogen were obtained. These are active materials, such as Ti-V-Zr-Ni, such as those described in US Pat. No. 4,551,400 ("patent 400"), issued by Sapru, Hong, Fetcenko and Venkatesan, the disclosure of which is incorporated by reference. These materials reversibly form hydrides in the order of accumulation of hydrogen. All materials used in the patent 400 use the original Ti- V-Ni composition, where at least one of Ti, V and Ni is present with at least one or more of Cr, Zr and Al. The materials of patent 400 are multiphase materials that may contain but are limited to one or more AB 2 phases with crystal structures, such as C 14 and C 15 . The following formulas are specifically described in patent 400:
(TiV 2-x Ni x ) 1-y M y ,
Where
X is between 0.2 and 1.0;
Y is between 0.0 and 0.2; And M-Al or Zr,
Ti 2-x Zr x V 4-y Ni y ,
Where
Zr is partially replaced by Ti;
X is between 0.0 and 1.5;
Y is between 0.6 and 3.5, and
Ti 1-x Cr x V 2-y Ni y ,
Where
Cr is partially replaced by Ti;
X is between 0.0 and 0.75;
Y is between 0.2 and 1.0.
Other Ti-V-Zr-Ni alloys can and can be used for a rechargeable negative electrode with hydrogen dissolution. Another such family of materials are those described in US Pat. No. 4,728,586 ("patent 586") issued to Venkatesan, Reichman and Fetcenko on "Alloys for electrochemical storage of hydrogen with increased charge retention and an electrochemical cell with increased charge retention", the description of which is included By mention. Patent 586 describes a specific subclass of these Ti-V-Ni-Zr alloys including Ti, V, Zr, Ni and the fifth component, Cr.
The most preferred embodiment of the alloy of patent 586 is composition
(Ti 2-x Zr x V 4-y Ni y ) 1-z Cr z ,
Where x is from 0.00 to 1.5;
Y is from 0.6 to 3.5;
Z is an effective amount less than 0.20.
These alloys can be considered stoichiometrically as containing 80 atm. % Of the content of V-Ti-Zr-Ni and up to 20 atm.% Cr, where the ratio (Ti + Zr + Cr + optional modifiers) to (Ni + V + optional modifiers) is between 0.40 and 0.67. Patent 586 contemplates the possibility of additives and modifiers, along with Ti, V, Zr, Ni and Cr alloy components, and generally discusses specific additives and modifiers, the amounts and interactions of these modifiers and the specific advantages that could be expected from them.
The family of V-Ti-Zr-Ni alloys described in patent 586 has a significantly higher discharge rate than the previously described alloys. This is the result of significantly higher surface areas at the metal / electrolyte interface for electrodes made of V-Ti-Zr-Ni materials. The roughness coefficient of the surface (total surface area divided by the geometric surface area) of the V-Ti-Zr-Ni alloys is approximately 10,000. This value indicates a very high surface area and is confirmed by an essentially high discharge rate of these materials. The characteristic surface roughness of the metal / electrolyte interface is the result of the disordered nature of the material. Since all the constituent elements and, like many of its alloys and phases, are represented in the volume of the metal, they are represented on the surfaces and in the cracks that form at the metal / electrolyte interface. Thus, the characteristic roughness of the surface is an important observable feature of the physical and chemical properties of the parent metals, as well as of the alloys and crystallographic phases of the alloys, in the alkaline environment. These microscopic chemical, physical, and crystallographic parameters of the individual phases in the material of the hydrogen storage alloy are supposed to be important in determining the macroscopic electrochemical characteristics.
In addition to the physical nature of their rough surfaces, it has been found that V-Ti-Zr-Ni alloys tend to achieve a stationary surface composition and particle size. The stationary composition of the surface is characterized by a relatively high concentration of metallic nickel. These observations are consistent with a relatively high rate of removal upon deposition of titanium and zirconium oxides from the surface and with a slower rate of solubilization of nickel providing a porosity level of the surface. The resulting surface appears to have a higher nickel concentration than would be expected from the volumetric composition of the negative electrode accumulating hydrogen. Nickel in the metallic state is the catalyst and conductor of electricity, giving these properties to the surface. As a result, the surface of the negative electrode accumulating hydrogen is the best catalyst and conductor than would be the surface containing a higher concentration of insulating oxides.
The surface of the negative electrode, which has conductive and catalytic components, such as metallic nickel, catalyzes numerous stages of the charge and discharge reaction.
In contrast to the Ti-V-Zr-Ni-based alloys described above, alloys such as AB 5 are usually considered as "ordered" materials that have a different chemistry and microstructure and exhibit different electrochemical characteristics compared to Ti-V-Zr- Ni alloys. However, the analyzes found that, although the early alloys of AB 5 could be ordered materials, the recently developed AB 5 alloys are not such. The characteristics of the early ordered materials AB 5 are poor. However, the currently used AB 5 alloys have a high level of modification (i.e., the number and number of modifying elements has increased) and the characteristics of the AB 5 alloys have improved significantly. This is caused by the disorder introduced by the modifier, and, like their electrical and chemical properties. The evolution of AB 5 alloys from a particular class of "ordered" materials to modern multi-component, multiphase "disordered" alloys that are very similar today to Ti-V-Zr-Ni alloys is presented in the following patents: (i) US Pat. No. 3,894,928 ; (Ii) U.S. Patent No. 4,214,043; (Iii) US Pat. No. 4,107,395; (Iv) US Pat. No. 4,107,405; (V) U.S. Patent No. 4,112,199; (Vi) US Pat. No. 4,125,588; (Vii) US Pat. No. 4,214,043; (Viii) U.S. Patent No. 4,216,274; (Ix) US Pat. No. 4,487,817; (X) US Pat. No. 4,605,603; (Xii) US Pat. No. 4,696,873; And (xiii) US Pat. No. 4,699,856. (These references are extensively discussed in US Pat. No. 5,096,667, and this discussion is specifically included by reference).
To put it simply, in AB 5 alloys, like Ti-V-Zr-Ni alloys, as the level of modification increases, the role of the initially ordered basic alloy becomes the role of the second plan in comparison with the properties and disorder associated with specific modifiers. In addition, the analysis of modern multicomponent AB 5 alloys shows that modern systems of AB 5 alloys are modified following the directions established for AB 2 systems. Thus, highly modified AB 5 alloys are identical AB 2 alloys in that they are both disordered materials that are characterized by a multicomponent and multiple phases, and there is no longer a significant difference between the two types of multicomponent, multiphase alloys.
Although, as is known in the literature, hydrogen storage alloys often contain various individual modifiers and combinations of modifiers to improve their performance, there is no clear teaching on the role of each individual modifier, the interaction of each modifier with other alloy components, or the effect of each modifier on specific operating parameters . Since heavily modified AB 5 alloys were analyzed in the context of the study of well-ordered crystalline materials, the effect of these modifiers, in particular, was not clearly understood.
Hydrogen-accumulating alloys known from the literature, as a rule, allow improving operating parameters such as the number of cycles, discharge rate, discharge voltage, polarization, self-discharge, capacity at low temperature and voltage at low temperature. However, alloys known from the literature give cells that exhibit quantitative improvement in one or two performance characteristics due to a quantitative deterioration in other performance characteristics. The often obtained performance characteristics of these cells are, at times, only slightly better than comparable characteristics of other types of cells, such as Ni-Cd cells. Thus, all cells produced from alloys known from the literature are cells for special purposes, their performance characteristics, both good and bad, represent an engineering compromise, and therefore are designed only for the intended use of the cell.
One of the objects of the present invention is hydrogen storage alloys which exhibit improved performance without impairing other performance characteristics.
Another object of the present invention is hydrogen storage alloys, which exhibit significantly improved performance compared to other types of alloys.
This and other objects of the present invention are satisfied by hydrogen storage accumulators having the composition:
(Main Alloy) a CO b Mn c Al d Fe e La f Mo g , where the Base Alloy is an unordered multicomponent alloy, an alloy having at least one structure selected from the group consisting of: amorphous; Microcrystalline; Polycrystalline (with missing composite long-range order, with three or more phases of said polycrystalline structure), and any combination of these structures; B is from 0 to 7.5 atm%, preferably from 4 to 7 atm%; C is from 0.1 to 8.5 atm.%, Preferably from 6 to 8 atm.%; D is from 0 to 2.5 atm.%, Preferably from 0.1 to 2 atm.%; E is from 0.1 to 6 atm.%, Preferably from 1 to 3 atm.%, Or from 5.3 to 6 atm.%; F is from 0 to 4.5 atm.%, Preferably from 1 to 4 atm.%, G is from 0 to 6.5 atm.%, Preferably from 0.1 to 6 atm. %, Most preferably about 6 atm.%; B + c + d + e + f + g> 0; And a + b + c + d + e + f + g = 100 atm.%.
Still other objects of the present invention are met by a metal hydride cell containing a negative electrode composed of the alloys described above.
 |
 |
 |
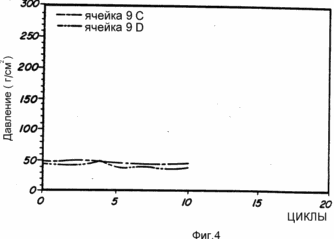 |
FIG. 1 to 4 show the capacitance versus the number of cycles, the pressure versus the number of cycles and the pressure from the over-current for various embodiments of the present invention.
The materials based on the disordered metal hydride alloys of the present invention are designed to produce unusual two- and three-dimensional configurations by varying the three-dimensional interactions of constituent atoms and their various orbitals. The disorder in these alloys is due to compositional, positional and translational relations, and both from the disorder created by the number, position and size of the crystallites of atoms, which are not limited by the usual crystalline symmetry in their freedom of interaction. This disorder can be of an atomic nature in the form of a composite or configuration disorder created throughout the volume or in various regions of the material. These disordered alloys have less order than the highly ordered crystal structures that provide single-phase materials, such as those used in many electrode alloys known from the literature. Types of disordered structures that create local structural chemical environments for improved hydrogen accumulation characteristics of the present invention are multicomponent polycrystalline materials with no long-range composite order: microcrystalline materials; Amorphous materials having one or more phases; Multiphase materials containing both amorphous and crystalline phases, or mixtures thereof.
The framework for disordered metal hydride alloys is the original matrix of one or more elements. The starting elements are chosen, as a rule, being hydride-forming, and they can be elements with a small atomic weight. Exemplary elements of the original matrix may be LaNi or TiNi. Elements of the original matrix are modified by incorporating selected modifying elements, which may or may not be hydride-forming.
We have found through numerous analyzes that, regardless of the starting materials of the parent matrix, when numerous modifying elements such as those described in the present invention are introduced, the resulting disordered material has excellent electrochemical properties due to the growth in the amount and spectrum of catalytically active Areas accumulating hydrogen. In particular, multi-orbital modifiers, for example, transition elements, provide a greatly increased number of storage areas due to the availability of a variety of communication configurations, thus resulting in an increase in energy density. The modification, which results in a nonequilibrium material having a high degree of disorder, provides unique communication configurations, overlapping of orbitals and, thus, a spectrum of free-bonded regions. Due to the different degrees of overlapping of orbitals and the disordered structure, a significant amount of structural rearrangements occur during charge / discharge cycles, or during periods of rest, resulting in a large number of cycles and a long storage time.
The accumulation of hydrogen and other electrochemical characteristics of the disordered electrode materials can be controlled in a controlled manner depending on the type and amount of material of the original matrix and the modifying elements chosen to produce the materials of the negative electrode. The alloys for the negative electrodes of the present invention are resistant to degradation by poisoning, due to the increased number of selectively designed accumulating and catalytically active sites that contribute to a large number of cycles. And some of the areas created in the material can bind and resist poisoning without affecting the regions active with respect to hydrogen. The materials obtained in this way have a very low self-discharge, and hence a good storage time.
As used herein, the term "Base Alloy" refers to a disordered alloy having a base alloy (as the term is described in patent 400), which is an unordered multicomponent alloy having at least one structure selected from the group consisting of amorphous, microcrystalline , Polycrystalline (with no long-range composite order and with three or more phases of said polycrystalline structure), and any combination of these structures. The terms "amorphous", "microcrystalline" and "polycrystalline" are used as defined in US Pat. No. 4,623,597 to Sapru, et al., The contents of which are incorporated by reference. The alloys of the present invention are not limited to any particular structure. These materials are classified as having an unordered structure and enclose materials that are commonly referred to using other diverse terms such as AB, AB 2 , AB 5 , LaNi 5 , misch metal, C 14 , C 15 , Laves phase, and so on. The most preferred composition of this Base Alloy contains 0.1 to 60 atm% Ti, 0.1 to 25 atm.% Zr, 0.1 to 57 atm.% Ni, and 0.1 to 56 atm% Cr .
The alloys of the present invention contain negative electrodes for metal hydride cells which exhibit an exceptionally high storage capacity, other significant quantitative improvements in their performance compared to cells known from the literature. Surprisingly, embodiments of this invention show an improvement in most, if not all, of their performance characteristics, and thus can be considered as cells of universal application.
According to the invention, it has been found that the alloys of the present invention described above and in the Summary of the Invention can further be classified as having an unordered microstructure where hydrogen in a separate phase is not discharged easily through either a small surface area or through an oxide with limited porosity or catalytic properties . Examples of this invention are shown in Table. 1 below.
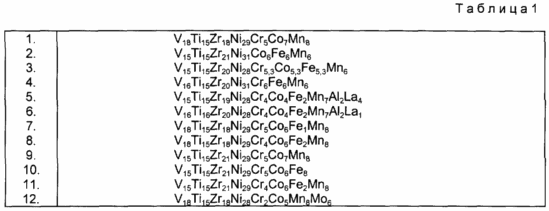
The effect of adding M can be seen in the materials for the negative electrodes of the present invention having the composition of formula I.
(Basic Alloy) a Co b Mn c Fe d ,
Where
B is from 0.1 to 7.5 atm.%, Preferably from 5 to 7.0 atm.%; C is from 0.1 to 8.5 atm.%, Preferably from 6.0 to 8.0 atm.%; D is from 0.1 to 6.5, preferably from 5.3 to 6 atmospheres, and a + b + c + d atm. %. Alloy N 3 is the embodiment of these materials.
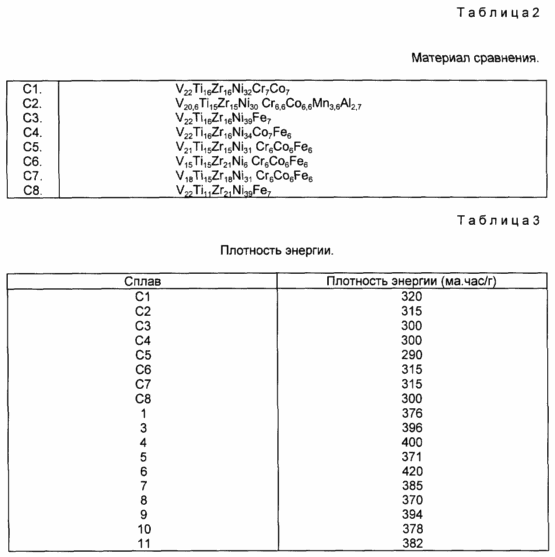
Comparison of the materials of formula (I) with the materials originally described demonstrates that using 6 to 8 atm% Mn results in increased capacity and performance at low temperature, and like low cell pressure and a large number of cycles. For example, alloy No. 3 has a capacity of 396 ma. H / g compared to the alloy N C6, the embodiment of materials known from the literature, which has an energy density of only 315 m.ppm.
Although not wishing to be bound by theory, it is believed that in the alloys of the present invention, Mn changes the microstructure in such a way that the precipitation of phases having hydrogen bond strengths outside the electrochemical fitness range is inhibited. One of the ways in which Mn apparently does this is to increase the mutual solubility of the other elements in the primary phases during hardening. In addition, Mn acts on the electrochemically active surface of the oxide as a catalyst. Multiple oxidation states of Mn are supposed to catalyze the electrochemical discharge reaction by increasing the porosity, conductivity and surface area of the active surfaces of the oxide film.
Another role of Mn is observed in materials for negative electrodes having composition 2
(Basic Alloy) a Co b Mn c
Where
B is from 4.0 to 7.5 atm.%, Preferably from 6.5 to 7.5 atm.%; S is from 5.5 to 8.5 atm.%, Preferably from 7.5 to 8.5 atm.%; And a + b + c = 100 atm.%. Alloys N 1 and N 9 are embodiments of these materials.
In the alloys of formula (2), the addition of Mn gives improved characteristics at a low temperature, and like an increased hydrogen storage capacity. For example, this can be replaced by comparing alloy No. 1, which has an energy density of 376 m.p./g or an alloy No. 9 that has an energy density of 395 m.p / g, with an N C7 alloy that has an energy density of 315 ma. H / g.
В дополнение, Mn может быть введен в качестве замещения для Fe в сплавах формулы (2). Хотя и не желая быть связанным теорией, предполагается, что, когда Mn представлен без Fe, Mn помогает электрохимической реакции разряда при низкой температуре, способствуя диффузии водорода при низкой температуре, а и путем катализа реакции водорода и ионов гидроксила на поверхности сплава. Из-за низкотемпературных свойств сплавов формулы (2) можно считать, что каталитические свойства Mn усиливаются, когда Fe не представлено или по крайней мере представлено только при низких концентрациях.
Другие эффекты материалов данного изобретения удовлетворяются с помощью электрохимической ячейки, содержащей отрицательный электрод, имеющий состав
(Основной Сплав) a Mn b Fe c ,
где b составляет от 5,5 до 6,5 атм.%; c составляет от 5,5 до 6,5 атм.%, предпочтительно приблизительно 6 атм.%; и a + b + c = 100 атм.%. Сплав N 4 является воплощением этих материалов.
В материалах формулы (3) Mn замещают Co. В материалах формулы (3) можно наблюдать, что емкость аккумулирования водорода увеличивается при сохраняющемся превосходном удерживании заряда. Это можно увидеть ниже, сравнивая сплав N 4, который имеет плотность энергии 400 ма.ч/г, со сплавом N С6, который имеет плотность энергии только 315 ма.ч/г.
Хотя и не желая быть связанным теорией, предполагается, что в сплавах формулы (3), подобно сплавам формулы (I), описанным выше, Mn изменяет микроструктуру и действует как катализатор на электрохимически активной поверхности оксида.
Другой аспект данного изобретения включает ячейки, имеющие отрицательные электроды, образованные из сплавов формулы 4 (Основной Сплав) a Co b Mn c Al d Fe e La f , где b составляет от 0,1 до 7,5 атм.%, предпочтительно от 5 до 7 атм.%; c составляет от 0,1 до 8,5 атм.%, предпочтительно от 7 до 8 атм.%; d составляет от 0,1 до 2,5 атм.%, предпочтительно приблизительно от 1,5 до 2,5 атм.%; e составляет от 0,1 до 3 атм.%, предпочтительно от 1 до 2 атм.%; f составляет от 0,1 до 4,5 атм.%, предпочтительно от 1 до 4 атм. %; и a + b + c + d + e + f = 100 амт.%. Сплавы N 5 и N 6 являются воплощениями этих материалов.
В состав материалов формулы (4) малая добавка La может быть полезной при увеличении емкости аккумулирования водорода и, как и емкости при низкой температуре. Это можно увидеть, сравнивая сплав N 5 со сплавом N С8. Смотри табл. 5, ниже.
В частности, мы замечаем, что чистота используемого La не является критичной для данного изобретения, и различные формы мишметалла, видимо, являются такими же эффективными как La высокой чистоты. Таким образом, как он используется здесь La включает La высокой чистоты и/или мишметалл, где редкоземельные компоненты мишметалла могут состоять из любого из многочисленных коммерчески доступных материалов, некоторые из которых могут содержать La в больших или малых количествах или вообще не содержать.
Хотя и не желая быть связанным теорией, предполагается, что добавление La имеет несколько функций;
(I) La функционирует в качестве гидрида. Хотя La в форме LaNi 5 адсорбирует заметное количество водорода, La в LaNi 5 легко окисляется и корродирует в щелочной среде. Однако эта коррозия не наблюдается в неупорядоченных сплавах данного изобретения. Предполагается, что упорядоченные составы данного изобретения, которые включают La, такие как те, что описаны упомянутой выше формулой, "защищают" La от коррозии без вмешательства в адсорбцию La водорода.
(2) La действует, удаляя примеси во время процесса плавления. Во время высокотемпературного плавления, как предполагается, La адсорбирует примеси, такие как кислород, поскольку он имеет высокую свободную энергию для образования оксидов. Предполагается, что кислород эффективно удаляется с участков трещин в стандартном сплаве, оставаясь в La фазах, обогащенных примесями, таким образом обеспечивая увеличенные участки аккумулирования во основном сплаве.
(3) La в более высоких концентрациях, видимо, способствует низкотемпературному разряду тем же самым способом, как он удаляет кислород. Заметно, что примеси легких элементов играют ключевую роль в ингибировании начальной диффузии водорода во время низкотемпературного разряда. Удаление этих примесей путем использования La или любого другого "приобретателя примесей" должно, таким образом, быть ключевым фактором в способствовании хорошему низкотемпературному разряду.
Quite unexpectedly, some preferred embodiments of the present invention, which contain from 0.1 to 3 atm.%, Preferably from 1 to 2 atm. % Fe and 6.5 to 8.5 atm.%, Preferably 7 to 8 atm.% Mn, exhibit a significantly improved charge retention compared to the literature data. In addition, these Core Alloys exhibit excellent low temperature performance, and like a significant increase in the number of cycles and other performance. Particularly preferred embodiments of these alloys are alloys of formula 5
(Basic Alloy) a Co b Mn c Fe d ,
Wherein b is from 4.5 to 7.5 atm.%, Preferably from 5 to 7 atm.%, C is from 6.5 to 8.5 atm.%, Preferably from 7 to 8 atm.%; D is 0.1 to 3 atm%, preferably 1 to 2 atm%, and a + b + c + d = 100. The alloy No. 7, No. 8, No. 10 and No. 11 are embodiments of these materials.
The materials of formula (5) have a very low operating pressure and exhibit a large number of cycles, a high discharge rate, a substantially increased hydrogen storage capacity, increased charge retention, and improved low-temperature discharge ability. This can be seen by comparing alloys N 7, N 8, N 10 and N 11 with known alloys, such as alloys N C5 and N C6 (see Tables 2-5 below).
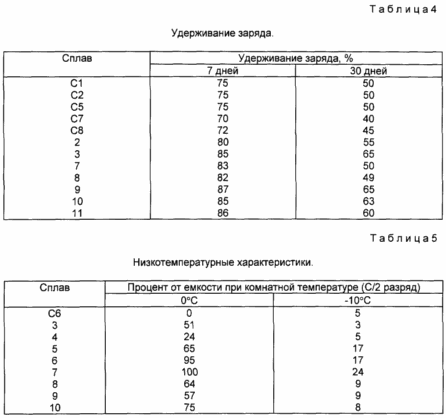
The improved performance of these formulations is accompanied by an unexpected discovery that the advantageous effects of the addition of Mn to the compositions of the present invention on the performance characteristics at a low temperature can be inhibited by the unoptimized amount of Fe. In particular, we have discovered that Fe in amounts of about 0.1 to 3 atm.% And more particularly 1 to 2 atm. %, Improves operation at a low temperature in comparison with similar alloys having Fe at about 6 atm%. We discovered that a lower amount of Fe still provides a predominant effect on the increased number of cycles. See Table. 5, below.
Advantageous effects of Mn and Fe are detailed in US Pat. No. 5,096,667, in US Pat. No. 5,104,617 and in US Patent Application No. 07 / 746,015 (pending), the contents of which are incorporated by reference.
US Pat. No. 5,104,617 notes that it is widely believed that the inclusion of Fe in metal hydride hydrogen storage materials should have a negative effect on the electrochemical characteristics. This assumption is caused by the information that Fe easily oxidizes and corrodes, especially in the presence of an alkaline electrolyte. Oxidation impairs the performance of the metal hydride electrode in various ways, and the Fe oxides, as is known in the literature, adversely affect the nickel-hydroxide positive electrode, especially with respect to the efficiency of the charge, and thus the capacity and number of cycles.
Still other embodiments of this invention contain negative electrodes comprising alloys of 6:
(Main Alloy) a Co b Mn c Mo d , where b is 0.1 to 5.5 atm%, preferably 4.5 to 5.5; C is 0.1 to 8.5 atm%, preferably 7.5 to 8.5 atm%, d is 0.1 to 6.5 atm%, preferably 5.5 to 6.5 Atm%, and a + b + c + d = 100. The alloy No. 12 is the embodiment of these materials.
All alloys of this invention contain Mn. The effects of adding Mn to these alloys are discussed, in general, in US Pat. No. 5,096,667, the contents of which are included by reference. The addition of Mn usually results in an increased charge efficiency. Although not wishing to be bound by theory, this effect is apparently the result of the ability of Mn to increase the efficiency of charging the alloys to which it is added by improving the resistance to oxidation and recombination of oxygen. It is observed that the gaseous oxygen released on the nickel-hydroxide positive electrode recombines on the surface of the metal hydride electrode. Oxygen recombination is a particularly aggressive oxidizer of its environment, even in comparison with an alkaline electrolyte.
It is possible that the modifying elements of the Base Alloy of the present invention, in particular Mn and Fe, and especially Co, either alone or in combination with Mn and / or Al, for example, act to catalyze the reduction of oxygen, thereby preventing or reducing Oxidation of surrounding elements in metal hydride alloy.
It is assumed that this function of modified alloys reduces or even eliminates the formation and development of harmful surface oxide, thereby providing a thin and more stable surface.
In addition to these effects, and quite unexpectedly, we found that the combination of Mn and excess Fe hinders the benefits of Mn in low-temperature characteristics, even if the discharge rate characteristics at room temperature can remain unchanged.
Although not wishing to be bound by theory, it is assumed that several additional factors can explain the unexpected properties of Mn and Fe in the Basic Alloy of the present invention:
(1) The combination of Mn and excess Fe can have an effect in the volume of the alloy, inhibiting the rate of bulk diffusion of hydrogen inside the metal by forming a complex phase structure, either by influencing grain boundaries, or by affecting the strength of the equilibrium hydrogen bond within the metal. In other words, the temperature dependence of the strength of the hydrogen bond can be increased, thereby reducing the available voltage and capacitance available at low temperature discharge.
(2) It is assumed that the combination of Mn and excess Fe can result in a lower surface area of the electrode for metallurgical reasons, increasing the ductility of the alloy and thereby reducing the amount of surface area formed during the activation process.
(3) It is assumed that the combination of Mn and excess Fe in these alloys can inhibit the low-temperature discharge by changing the oxide layer itself with respect to conductivity, porosity, thickness and / or catalytic activity. The oxide layer is an important factor in the discharge reaction and promotes the reaction of hydrogen from the Base Alloy of the present invention and the hydroxide ion from the electrolyte. We assume that this reaction is facilitated by a thin, conductive, porous oxide having some catalytic activity.
The combination of Mn and excess Fe is not, apparently, a problem when discharged at room temperature, but exhibits an unexpected tendency to slow the low-temperature reaction. The formation of a complex oxide can result in a subtle change in the oxide structure, such as porosity or pore size distribution. Since the discharge reaction produces water on the metal hydride surface and inside the oxide itself, a small pore size can cause the slow diffusion of K + and OH ions from the electrolyte volume to the oxide. When discharged at room temperature, where the polarization is almost entirely ohmic, and when the discharge is at a low temperature, where the activation and concentration components of the polarization dominate, the physical structure of oxides with Fe and Mn can be significantly different in comparison with only M-based oxides.
Another possible explanation is that Mn and Fe have oxidation states with different valencies. It is considered that some elements inside the oxide can actually change oxidation states through the normal state of charge change as a function of speed and discharge, and depend both on temperature and on the mode of production, can be determined by composition. Perhaps these states with different degrees of oxidation have different catalytic activity, and like different densities, which together affects the porosity of the oxide.
A possible problem with a complex oxide containing both Mn and excess Fe may be that the Fe component slows the ability of Mn to change the oxidation state, if present in large amounts.
Concerning the previous discussion with respect to the oxide, it should be noted that the oxide also contains other components of the Base Alloy of the present invention, such as, V, Ti, Zr, Ni and / or Cr and other modifying elements. Discussion of complex oxide Mn and Fe is carried out only for the sake of brevity, and specialists should not consider that this mechanism can not include another or more complex explanation including other such elements.
Negative electrodes using the alloys of this invention can be used in various types of cells and batteries with hydrogen storage. These include flat cells having a sufficiently flat negative electrode, a gasket and a positive electrode or a measuring electrode that is substantially flat and positioned so as to be in operative contact with the negative electrode; Roulet cells obtained by spirally winding a flat cell about an axis; And prismatic cells for use, for example, in electric vehicles. The metal hydride cells of the present invention can use a suitable type of container and can be constructed, for example, from metal or plastic.
An aqueous solution of 30% by weight potassium hydroxide is the preferred electrolyte.
In a particularly preferred embodiment, alloys used in conjunction with advanced gasket materials as described in pending US patent application Ser. No. 07 / 879,823, entitled "Metal-hydroxide cells having an increased number of cycles and charge retention," by Fetcenko et al. , Give improved characteristics of alloys for certain electrochemical applications as compared to the literature data.
Along with the improved technical characteristics discussed above, the alloy modification gives an advantage in cost up to 30%. One of the dominant factors affecting the cost of the base alloy is the cost of metallic vanadium. In US Pat. No. 5,002,730, incorporated by reference, vanadium in the form of V-Ni or V-Fe provides significant cost advantages over pure vanadium. Such cost improvements can be increased in the Base Alloys of the present invention by using V-Fe.
EXAMPLES OF OBTAINING MATERIALS OF NEGATIVE ELECTRODES
Materials of alloys, described in Table. 1 above and the comparative materials described in Table 1. 2 are prepared and fabricated, as described below, into the materials of the negative electrodes. The particular alloys that are used refer to the tables in each specific example. The numbering of alloys is consistent throughout the application and is listed in Table. 1 or table. 2.
Alloys in Table. 1 and 2 are prepared by weighing and mixing the constituent element materials in a graphite crucible, as described in US Pat. No. 5,002,230 to Fetcenko and 4,948,423 to Fetenco, etal. The crucible and its contents are placed in a vacuum oven which is pumped out and then filled with argon under a pressure of approximately one atmosphere. The content of the crucible is melted by high-frequency induction heating, leaving it in an argon atmosphere. The melting is carried out at a temperature of about 1500 ° C. until a uniform melt is obtained. At this time, the heating is stopped and the melt is allowed to solidify in a confined space under an inert atmosphere.
The ingot of the alloy material is then reduced in size in a multi-stage process. The first step involves the hydridization / dehydrogenation process, generally as described in US Pat. No. 4,983,756 to Fetcenko, etal., Entitled "Device-Hydride Reactor for the Hydrogen Chopping of an Alloy Material for Metal-Hydride Hydrogen Storage," the description of which is specifically incorporated by reference. In this first stage, the alloy is reduced in size to less than 100 mesh. After this, the material obtained during the hydridization / dehydrogenation process is further reduced in size by means of a pulsed grinding process in which the particles are tangentially and radially accelerated with respect to the impulse block. The method is described in U.S. Patent No. 4,915,898 to Wolff, et al. , Entitled "Method for the continuous production of ground material of a negative electrode based on an alloy for the accumulation of hydrogen," the description of which is specifically included by reference.
The alloy material fraction having a particle size of less than 200 mesh and a weight average particle size of about 400 mesh (38 microns) is extracted from the pulverized milling process and bonded to the nickel mesh current collector in a manner that includes placing the alloy material layer on the current collector and pressing Powder and collector. The pressing is carried out in an inert atmosphere in two separate pressing stages, each at a pressure of about 2.481 tons per square centimeter. After pressing, the current collector and the powder adhering to it are sintered in an atmosphere of approximately 2 atm% hydrogen, forming the materials of the negative electrodes.
These alloys and negative electrodes are activated using the alkaline etching treatment described in US Pat. No. 4,716,088 to Reichmann, et al., The disclosure of which is specifically incorporated by reference. Practically some oxidation takes place during the fabrication of the electrode, and therefore, opening the alloy powder or negative electrodes of the present invention to the alkaline solution, in order to "etch" or change the nature of the surface oxides that are formed, a variety of beneficial results are obtained. For example, it is assumed that etching changes the surface state of the alloy powder or formed negative electrode in such a way that an increase in charge efficiency or even a first charging cycle is achieved; Promotes the ionic diffusion required for the electrochemical discharge process, creates a gradient of the oxidation state on the surface of the material and changes the surface osmium, giving a greater susceptibility to the charge.
PREPARING CELLS
The prepared negative electrodes are collected with nickel hydroxide positive electrodes in sealed cells "C" having a sealable opening as described in US Pat. No. 4,822,377 to Wolff using 30% KOH electrolyte.
Example 1
The finished cells, prepared as described above, using the alloys shown in Table 1. 3 below, subjected to the conditions of charge and discharge and determine the Energy Density (mAh / g).
Data obtained from these tests are presented in Table. 3 below.
Example 2
The cells are prepared as described above, using the alloys listed in Table 1. 4, and the charge retention is determined.
The data obtained from these tests are presented in Table. 4 below.
Example 3
The cells are prepared as described above, using the alloys listed in Table 1. 5. The completed cells are subjected to charging and discharge conditions at low temperatures, and their capacity is measured.
The data obtained from these tests are presented in Table. 5 below.
Example 4
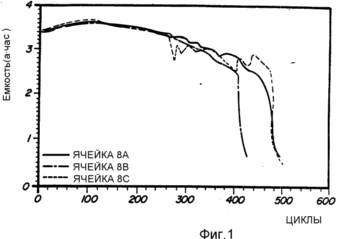
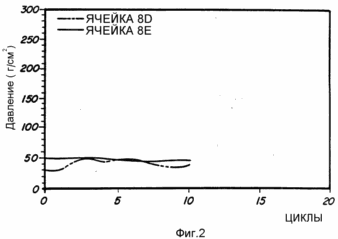
Cells 8A, 8B, 8C, and 8D and 8E are prepared as described above from No. 8 alloys. These representative cells are subjected to charge and discharge conditions. The results of this analysis are shown in FIG. 1 and 2. Fig. 1 shows a peak capacity of about 3.7 Ah and a life time of 500 cycles. FIG. 2 shows the pressure remaining constant during cycling.
Example 5
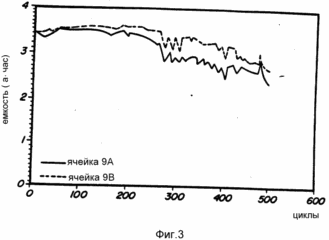
Cells 9A, 9B, 9C, 9D and 9E are prepared as described above from alloy No. 9. These representative cells are subjected to charge and discharge conditions. The analysis of the resulting cells is shown in Fig. 3 and 4. FIG. 3 shows a peak capacity of about 3.7 Ah and a life time of about 500 cycles. FIG. 4 shows the pressure remaining constant during cycling.
Typically, the compared alloys have some good overall performance in conjunction with some poor performance. The result is alloys that do not have performance characteristics that would work under all circumstances. This effectively limits their usefulness in specific applications for special purposes. For example, Alloy N C2 has good speed characteristics, but poor charge retention and a moderate number of cycles. Similarly, Alloy N C6 exhibits good energy density and good charge retention, but it requires a long activation, has a moderately high discharge rate and low temperature limitations.
In contrast, the alloys of the present invention have improved energy density, charge retention, and low temperature characteristics. The resulted alloys demonstrate that the Base Alloy of the present invention is very close to a universal alloy that can be used in a wide variety of configurations for a wide variety of applications.
In view of the foregoing, it is understood by those skilled in the art that the present invention defines and encompasses a number of alloy compositions that, when included as a negative electrode in metal hydride cells, result in batteries having improved performance.
The drawings, discussions, descriptions and examples of this description are exclusively illustrated for specific embodiments of the invention and are not to be construed as limitations on their use. The following is a claim that includes all equivalents that define the scope of the invention.
CLAIM
1. An unordered alloy for the electrochemical accumulation of hydrogen, comprising a base alloy a Co b Mn c Al d Fe e La f Mo g , wherein the base alloy contains 0.1-60 atm% Ti, 0.1-25 atm% Zr, 0.1-60 atm.% V, 0.1-57 atm.% Ni and 0.1-56 atm. % Cr, the base alloy being an unordered multicomponent alloy having at least one structure selected from the group consisting of: amorphous, microcrystalline, polycrystalline with an absence of long-range composite order, with three or more phases of polycrystalline structures, characterized in that for Any combination of these structures b is up to 7.5 atm%, c is from 0.1 to 8.5 atm. %, D is up to 2.5 atm%, e is from 0.1 to 6 atm. %, F is up to 4.5 atm%, g is up to 6.5 atm%, b + c + d + e + f + g> 0 and a + b + c + d + e + f + G = 100 atm.%.
2. The alloy according to claim 1, characterized in that b is from 4 to 7 atm%, c is from 6 to 8 atm%, d is from 0.1 to 2 atm%, e is from 1 to 2 At%, f is from 0.1 to 4 atm%, g is from 0.1 to 6 atm. %.
3. The alloy according to claim 1, characterized in that the alloy has the following composition: a base alloy a Co b Mn c Fe d , where b is up to 7.5 atm%, c is from 0.1 to 8.5 atm .%, D is from 0.1 to 6 atm.%, A + b + c + d = 100 atm.%.
4. An alloy according to claim 5, characterized in that b is from 5.3 to 7.0 atm%, c is from 6.0 to 8.0 atm%, and d is from 1.0 to 2.0 At%.
5. An alloy according to claim 1, characterized in that the alloy has the following composition: a base alloy a Co b Mn c where b is from 4.0 to 7.5 atm%, c is from 5.5 to 8.5 Atm% and a + b + c = 100 atm.%.
6. An alloy according to claim 7, characterized in that b is from 6.5 to 7.5 atm%, c is from 7.5 to 8.5 atm.%.
7. An alloy according to claim 1, characterized in that the alloy has the following composition: a base alloy a Mn b Fe c , where b is from 5.5 to 6.5 atm%, c is from 5.5 to 6.5 Atm% and a + b + c = 100 atm.%.
8. The alloy according to claim 1, characterized in that the alloy has the following composition: a base alloy a Co b Mn c Al d Fe e La f , where b is from 0.1 to 7.5 atm%, c is from 0 , 1 to 8.5 atm.%, D is 0.1 to 2.5 atm.%, E is 0.1 to 3 atm.%, F is 0.1 to 4.5 atm.%, And A + b + c + d + e + f = 100 atm.%.
9. An alloy according to claim 8, characterized in that b is from 5 to 7 atm%, c is from 7 to 8 atm%, d is from 1.5 to 2.5 atm%, e is from 1 Up to 2 atm.%, F is from 1 to 4 atm.% And a + b + c + d + e + f = 100 atm.%.
10. An alloy according to claim 1, characterized in that the alloy has the following composition: a base alloy a Co b Mn c Fe d , where b is 4.5 to 7.5 atm%, c is from 6.5 to 8 , 5 atm%, d is from 0.1 to 3 atm% and a + b + c + d = 100 atm.%.
11. An alloy according to claim 10, characterized in that b is from 5 to 7 atm%, c is from 7 to 8 atm.%, D is from 1 to 2 atm.%.
12. The alloy according to claim 1, characterized in that the alloy has the following composition: a base alloy a Co b Mn c Mo d , where b is from 0.1 to 5.5 atm%, c is from 0.1 to 8 , 5 atm%, d is from 0.1 to 6.5 atm.%, And a + b + c + d = 100 atm.%.
13. An alloy according to claim 12, characterized in that b is from 4.5 to 5.5 atmospheres. %, C is from 7.5 to 8.5 atm.%, D is from 5.5 to 6.5 atm.%.
14. An alloy according to claim 1, characterized in that the alloy has the following composition: V 12 Ti 5 Zr 18 Ni 29 Cr 5 Co 7 Mn 8 .
15. An alloy according to claim 1, wherein the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 31 Co 6 Fe 6 Mn 6 .
16. An alloy according to claim 1, wherein the alloy has the following composition: V 15 Ti 15 Zr 20 Ni 28 Cr 5.3 Co 5.3 Fe 5.3 Mn 6 .
17. An alloy according to claim 1, wherein the alloy has the following composition: V 16 Ti 15 Zr 20 Ni 31 Cr 6 Fe 6 Mn 6 .
18. An alloy according to claim 1, wherein the alloy has the following composition: V 15 Ti 15 Zr 19 Ni 28 Cr 4 Co 4 Fe 2 Mn 7 Al 2 La 4 .
19. An alloy according to claim 1, wherein the alloy has the following composition: V 16 Ti 16 Zr 20 Ni 28 Cr 4 Co 4 Fe 2 Mn 7 Al 2 La 1 .
20. The alloy of claim 1, wherein the alloy has the following composition: V 18 Ti 15 Zr 18 Ni 29 Cr 5 Co 6 Fe 1 Mn 8 .
21. An alloy according to claim 1, wherein the alloy has the following composition: V 18 Ti 15 Zr 18 Ni 29 Cr 4 Co 6 Fe 2 Mn 8 .
22. The alloy according to claim 1, wherein the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 29 Cr 5 Co 7 Mn 8 .
23. An alloy according to claim 1, wherein the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 29 Cr 5 Co 6 Fe 1 Mn 8 .
24. An alloy according to claim 1, wherein the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 29 Cr 4 Co 6 Fe 2 Mn 8 .
25. The alloy of claim 1, wherein the alloy has the following composition: V 18 Ti 15 Zr 18 Ni 28 Cr 2 Co 5 Mn 8 Mo 6 .
26. A cell for electrochemical storage of hydrogen, comprising a positive electrode, a spacer, a negative electrode consisting of an unordered electrochemical alloy having the following composition: a base alloy a Co b Mn c Al d Fe e La f Mo g .
The base alloy is an unordered multicomponent alloy having at least one structure selected from the group consisting of amorphous, microcrystalline, polycrystalline structures with a missing long-range composite order, with three or more phases of the polycrystalline structure, characterized in that in any combination of these Structures b is up to 7.5 atm%, c is 0.1 to 8.5 atm%, d is up to 2.5 atm%, e is 0.1 to 6 atm.%, F Is up to 4.5 atm%, g is up to 6.5 atm%, b + c + d + e + f + g> 0 and a + b + c + d + e + f + g = 100 At%.
27. The cell of claim 26, wherein the base alloy comprises 0.1 to 60 atmospheres. % Ti, 0.1 to 25 atm.% Zr, 0.1 to 60 atm.% V, 0.1 to 57 atm.% Ni, and 0.1 to 56 atm.% Cr.
28. The cell of claim 26, wherein b is 4 to 7 atm%, c is 6 to 8 atm%, d is 0.1 to 2 atm%, e is 1 to 2 At%, f is 0.1 to 4 atm%, g is 0.1 to 6 atm.%.
29. The cell of claim 26, wherein the negative electrode further includes an unordered microstructure where the hydrogen in a particular phase is not discharged easily through either a small surface area or through an oxide with limited porosity or catalytic properties.
30. The cell of claim 26, characterized in that the alloy has the following composition, the base alloy a Co b Mn c Fe d , where b is up to 7.5 atm%, c is from 0.1 to 8.5 atm. %, D is from 0.1 to 6 atm.% And a + b + c + d = 100 atm.%.
31. The cell of claim 30, wherein b is from 5.3 to 7.0 atm%, c is 6.0 to 8.0 atm%, d is 1.0 to 2.0 At%.
32. The cell of claim 26, characterized in that the alloy has the following composition: a base alloy a Co b Mn c where b is from 4.0 to 7.5 atm%, c is from 5.5 to 8.5 Atm% and a + b + c = 100 atm.%.
33. A cell according to claim 26, characterized in that b is from 6.5 to 7.5 atm%, c is from 7.5 to 8.5 atm.%.
34. The cell of claim 26, characterized in that the alloy has the following composition: a base alloy a Mn b Fe c , wherein b is 5.5 to 6.5 atm%, c is 5.5 to 6.5 Atm% and a + b + c = 100 atm.%.
35. The cell of claim 26, characterized in that the alloy has the following composition: a base alloy a Co b Mn c Al d Fe e La f , where b is from 0.1 to 7.5 atm%, c is from 0 , 1 to 8.5 atm.%, D is 0.1 to 2.5 atm.%, E is 0.1 to 3 atm.%, F is 0.1 to 4.5 atm.%, And A + b + c + d + e + f = 100 atm.%.
36. The cell of claim 35, wherein b is 5 to 7 atm%, c is 7 to 8 atm%, d is 1.5 to 2.5 atm%, e is from 1 Up to 2 atm.%, F is from 1 to 4 atm.%.
37. A cell according to claim 26, characterized in that the alloy has the following composition: a base alloy a Co b Mn c Fe d , where b is 4.5 to 7.5 atm%, c is from 6.5 to 8 , 5 atm%, d is from 0.1 to 3 atm% and a + b + c + d = 100.
38. The cell of claim 37, wherein b is 5 to 7 atm%, c is 7 to 8 atm%, and d is 1 to 2 atm%.
39. A cell according to claim 26, characterized in that the alloy has the following composition: a base alloy a Co b Mn c Mo d , where b is from 0.1 to 5.5 atm%, c is from 0.1 to 8 , 5 atm%, d is from 0.1 to 6.5 atm.%, And a + b + c + d = 100.
40. The cell of claim 39, wherein b is 4.5 to 5.5 atm%, c is 7.5 to 8.5 atm%, d is 5.5 to 6.5 At%.
41. The cell of claim 26, wherein the alloy has the following composition: V 18 Ti 15 Zr 18 Ni 29 Cr 5 Co 7 Mn 8 .
42. A cell according to claim 26, characterized in that the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 31 Co 6 Fe 6 Mn 6 .
43. The cell of claim 26, characterized in that the alloy has the following composition: V 15 Ti 15 Zr 20 Ni 28 Cr 5.3 Co 5.3 Fe 5.3 Mn 6 .
44. A cell according to claim 26, characterized in that the alloy has the following composition: V 16 Ti 15 Zr 20 Ni 31 Cr 6 Fe 6 Mn 6 .
45. A cell according to claim 26, characterized in that the alloy has the following composition: V 15 Ti 15 Zr 19 Ni 28 Cr 4 Co 4 Fe 2 Mn 7 Al 2 La 4 .
46. A cell according to claim 26, characterized in that the alloy has the following composition: V 16 Ti 16 Zr 20 Ni 28 Cr 4 Co 4 Fe 2 Mn 7 Al 2 La 1 .
47. A cell according to claim 26, characterized in that the alloy has the following composition: V 18 Ti 15 Zr 18 Ni 29 Cr 5 Co 6 Fe 1 Mn 8 .
48. The cell of claim 26, characterized in that the alloy has the following composition: V 18 Ti 15 Zr 18 Ni 29 Cr 4 Co 6 Fe 2 Mn 8 .
49. The cell of claim 26, characterized in that the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 29 Cr 5 Co 7 Mn 8 .
50. A cell according to claim 26, characterized in that the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 29 Cr 5 Co 6 Fe 1 Mn 8 .
51. A cell according to claim 26, characterized in that the alloy has the following composition: V 15 Ti 15 Zr 21 Ni 29 Cr 4 Co 5 Fe 2 Mn 2 .
52. The cell of claim 26, wherein the alloy has the following composition: V 18 Ti 15 Zr 18 Ni 28 Cr 2 Co 5 Mn 8 Mo 6 .
53. The cell of claim 26, wherein the alloy is subjected to alkaline etching when it is in the form of a powder.
print version
Date of publication 28.02.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.