|
Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2281154
![]()
CATALYTIC COMPOSITE MATERIAL FOR STORAGE OF HYDROGEN
AND METHOD FOR STORING HYDROGEN IN CATALYTIC SYSTEMS BASED ON HYDROGENATION-DEHYDROGENATION OF ORGANIC COMPOUNDS
The name of the inventor: Bogdan Viktor Ignatievich (RU); Kustov Leonid Modestovich (RU); Kustov Arkady Leonidovich (RU); Tarasov Andrey Leonidovich
The name of the patent holder: Limited Liability Company "ENVAYROKET"
Address for correspondence: 121165, Moscow, PO Box 15, LLC "PPF-YUSTIS", Pat. L.S. Pilishkina
Date of commencement of the patent: 2004.04.30
The invention relates to the development of methods for storing hydrogen in catalytic systems operating on the basis of cyclic hydrogenation-dehydrogenation reactions of condensed and polynuclear aromatic compounds that can be used in hydrogen generators for industrial plants, fuel cells used in automobiles, and other devices and means , Equipped with hydrogen engines, or in power plants. The catalytic composite material for hydrogen storage contains as an hydrogen source an organic substrate capable of reversible hydrogenation and dehydrogenation. As a catalyst, the material comprises a heterogeneous catalyst comprising a carbon or oxide carrier with a high specific surface area with a metal VIII (platinum) group supported on this surface, with a substrate to catalyst ratio of 10: 1 to 1000: 1. As an organic substrate, it contains aromatic hydrocarbons: condensed, polycyclic, polyunsaturated, aromatic oligomers and polymers: biphenyl or its functional derivative, or terphenyl, naphthalene, or anthracene, or a functional derivative of one or the other, polystyrene or a copolymer thereof, polyacetylene or polycubulene. The method for storing hydrogen is to charge hydrogen at an elevated pressure of the described catalytic composite material and recover hydrogen therefrom by heating it under reduced pressure. The dressing is carried out by contacting the organic substrate and the heterogeneous catalyst at a temperature of from 50 to 180 ° C and a hydrogen pressure of 1 to 100 atm, and hydrogen evolution is carried out by contacting the hydrogenated substrate when refueling with the same catalyst at a temperature of 200 to 350 ° C, Atmospheric pressure.
DESCRIPTION OF THE INVENTION
The invention relates to the field of catalysis and organic chemistry, in particular to the development of methods for storing hydrogen in catalytic systems operating on the basis of cyclic hydrogenation-dehydrogenation reactions of condensed and polynuclear aromatic compounds, which can be used in hydrogen generators for industrial plants, fuel cells used In the car, as well as other devices and facilities equipped with hydrogen engines, or in power plants.
Hydrogen is widely used in the chemical, metallurgical and a number of other industrial industries. It is one of the main sources of energy for the future. The problems of the technological plan and cost, along with the availability and low cost of natural gas, gasoline and other raw materials of natural origin, limit the commercial use of hydrogen energy on the world market. Although the cost of hydrogen is currently three times higher than the cost of gasoline, given the environmental aspect of its use for transport purposes, the use of hydrogen as fuel in the near future will come to the fore. Hydrogen can become an alternative fuel if the problem of the functioning of its reversible storage (storage) and utilization systems is solved. The technical solution to these problems is being intensively pursued by the largest automobile concerns. The limiting factor in the use of hydrogen as motor fuel is the creation of storage systems. The use of liquid hydrogen is impossible because of the high cost of its production and the temperature requirements for its storage. Compressed hydrogen is much less expensive, but it requires large capacities for storage and is dangerous to use.
The discovery of materials capable of accumulating large amounts of hydrogen per unit volume or weight is currently the subject of research by a number of laboratories and research centers.
In recent years, a large number of papers have appeared in which carbon carriers are considered as hydrogen adsorbents. Theoretical calculation showed that carbon nanomaterials (nanotubes) are able to accumulate up to 4.1% hydrogen. This has been experimentally confirmed in a number of works (for example, J. Dillon, Storage of hydrogen in single wall carbon nanotubes, Nature, 1997, v.386, p. 377-379). However, studies show that to ensure maximum capacity for hydrogen, the cooling temperature of systems based on carbon nanomaterials should not be higher than -120 ° C. Cryogenic use conditions are a significant drawback of such systems.
Some progress has been made with the use of hydrides of a number of metals. There are a number of patents (eg, US Pat. No. 5,199,972) claiming the benefits of using such compounds as hydrogen storage systems and even for technical vehicle solutions (US Pat. No. 6,182,717).
Studies of the National Laboratory in Los Alamos (Schwarz, 1998) have shown that one of the most promising materials is magnesium hydride. It is of interest because it can store 7.7% of hydrogen, but the kinetics of hydrogen adsorption / desorption is much slower for it than for other hydrides, and rather high temperatures are required to extract hydrogen.
In the course of these studies, highly dispersed Mg 2 Ni materials were obtained, obtained by mechanical mixing and grinding in ball mills that catalyze hydrogen dissociation, while substantially increasing the rate of hydrogen adsorption, so that it becomes comparable with the adsorption rate for FeTi and LaNi 5 ( U.S. Patent No. 6,165,663). However, the limiting capacity for hydrogen for such a system does not exceed 5% by weight, and the temperature range of desorption is rather narrow and shifted to the high-temperature region.
Some hydrides, when decomposed, can release even more hydrogen, for example LiH up to 12.7% or LiAlH 4 to 10.6% (US Pat. No. 5,702,491). The rapid reaction of hydrolytic decomposition of metal hydrides with water makes the process of hydrogen accumulation irreversible. Thus, these systems are not regenerative, too expensive and can not compete with reversible systems.
In terms of the use of metal hydrides in the automotive industry, due to the low capacity of most such systems for hydrogen, large-sized devices for refueling should be used, otherwise the mileage of the engines will be very small. In addition, for reversible extraction of bound hydrogen hydrides should be heated to temperatures above 300 ° C. All this limits the use of such hydrogen storage systems.
The closest to the present invention is the hydrogen storage method described in US Pat. No. 6,074,447. A method for storing and recovering hydrogen fuel consists in using a mixture of a hydrogenated hydrocarbon and a homogeneous catalyst that, when heated to 190 ° C., releases hydrogen. As the catalyst, an iridium complex of the composition IrH 4 (2,6-C 6 H 3 (CH 2 P (C (CH 3 ) 3 ) 2 ) 2 )} is claimed. The catalyst concentration is 0.01-0.1%. As the material (substrate) that is subjected to dehydrogenation, hydrocarbons of the cycloalkane class are declared: methylcyclohexane, decalin, dicyclohexyl, cyclohexane or a combination thereof. The patent states that it is possible to reverse the process of regeneration of hydrogenated material at temperatures above 100 ° C and pressures above 10 atm.
A disadvantage of this method is the use of a difficult-to-obtain, expensive iridium complex and the need to use filters (or special cut-off devices) to separate the evolved hydrogen from the reaction mixture, since the boiling point of a number of the claimed substrates (eg methylcyclohexane or cyclohexane) is substantially lower than the process temperature. A disadvantage of this method is the impossibility of separating the homogeneous catalyst from the substrate, if necessary, for example, for regeneration. The application does not provide kinetic data (the rate of hydrogen evolution from a unit of volume), indicating the effectiveness of the method, as well as data on the number of hydrogenation-dehydrogenation cycles when used for hydrogen storage. It is obvious that the capacity of such a system for hydrogen continuously decreases from cycle to cycle and the use of such a homogeneous system and low-boiling substrates is ineffective in materials for storing hydrogen.
For the hydrogenation of aromatic hydrocarbons, catalysts based on platinum group metals (Pt, Pd) deposited on various carriers - Al 2 O 3 , aluminosilicates, etc., are widely used. Thus, a method for the preparation of an aromatic hydrocarbon hydrogenation catalyst consisting of introducing Pt or Pd into a matrix Aluminosilicates, such as ZSM-5 (U.S. Patent No. 5,874,622), or a process for the preparation of catalysts for the hydrogenation of butadiene-styrene copolymers (US Pat. No. 5,948,869). Dehydrogenation of paraffinic and cycloparaffinic hydrocarbons is carried out using the same catalysts containing noble metals (see, for example, US Pat. No. 5,672,801). Most patents are devoted to the hydrogenation and dehydrogenation of such simple hydrocarbons as benzene, cyclohexane or their derivatives. Reactions are carried out mainly in the vapor-gas phase. Examples of reactions in the liquid phase using polycyclic hydrocarbons are limited.
A technical problem solved in the present invention is the creation of an effective composite catalyst system and a method for storing and recovering hydrogen based on reversible hydrogenation-dehydrogenation cycles of organic compounds under the action of heterogeneous catalysts based on platinum group metals. The technical result of the catalytic composite system and the method for storing hydrogen with its use is to enable multiple refilling and hydrogen evolution at high speed.
Said technical result is achieved by the fact that a catalytic composite material for hydrogen storage containing as an hydrogen source an organic substrate capable of reversible hydrogenation and dehydrogenation and a hydrogenation-dehydrogenation catalyst according to the invention contains, as an organic substrate, an aromatic hydrocarbon selected from the group : Condensed, polycyclic, polyunsaturated, aromatic oligomers and polymers, and as the catalyst contains a heterogeneous catalyst comprising a carbon or oxide carrier with a high specific surface area coated with at least one metal of the VIII (platinum) group at a substrate mass ratio And a catalyst of from 10: 1 to 1000: 1.
In this case, as a polycyclic aromatic hydrocarbon, it can contain biphenyl or a functional derivative thereof, or terphenyl, and as condensed aromatic hydrocarbon, naphthalene or anthracene, or a functional derivative of one or the other.
As an aromatic polymer, the material can also contain polystyrene or a copolymer thereof with an average molecular weight of up to 1000, or polyacetylene, or polycubulene.
As a carbon carrier, the material may contain activated carbon, and as the oxide carrier, silica or alumina.
As the Group VIII metal, the material comprises platinum or palladium or nickel or a platinum-palladium alloy in an amount of 0.1 to 15% by weight of the heterogeneous catalyst.
The technical result is achieved by the fact that in a method for storing hydrogen by charging hydrogen at an elevated pressure of a catalytic composite material containing as an hydrogen source an organic substrate capable of reversible hydrogenation-dehydrogenation reaction and a hydrogenation-dehydrogenation catalyst and hydrogen evolution from the catalytic composite material When it is heated under reduced pressure, according to the invention, any of the materials described above are used as the catalytic composite material, it is charged by contacting an organic substrate and a heterogeneous catalyst at a temperature of 50 to 180 ° C. and a hydrogen pressure of 1 to 100 atm, and The hydrogen evolution is carried out by contacting the organic substrate hydrogenated when refueling with the same catalyst at a temperature of 200 to 350 ° C. at atmospheric pressure.
At the same time, it is expedient to carry out refueling and hydrogen recovery without mixing the composite material.
According to the proposed method, the step of hydrogen charging the system by hydrogenating the polycyclic aromatic compounds (or other organic substrates) and the hydrogen evolution step by dehydrogenating the corresponding hydrogen-forming (eg, polycycloparaffin) compounds is carried out in the presence of heterogeneous catalysts containing platinum, palladium or nickel supported On various carriers with a high specific surface, with the following technological parameters.
According to the invention, the heterogeneous catalyst contains from 0.1 to 15% by weight of the active metal, preferably from 0.5 to 5% by weight. Catalysts containing platinum, palladium or nickel, or other platinum metals or a mixture thereof, are prepared by impregnation (according to the moisture capacity) of various carbon or oxide carriers with a high specific surface area (activated carbon, graphitized Cibunite-type carbons, silicas or aluminum oxides) with aqueous solutions of salts or Complexes of active metals, for example Ni (NO 3 ) 2 , H 2 PtCl 6 or H 2 PdCl 4 , followed by drying in air at 100-150 ° C and reduction in hydrogen flow at 100-400 ° C, preferably at 200-300 ° C FROM. Even at the stage of preparation of catalysts, highly dispersed particles of noble metals or nickel are formed on their surface, which are highly active in hydrogenation and dehydrogenation reactions. The smallest fraction of carriers (particle size 5-100 μm) is used to provide a stable suspension of the catalyst in the substrate substance, which is necessary to ensure high rates of hydrogenation / dehydrogenation reactions even without stirring. It is preferable to use platinum or platinum alloys with palladium as the active component because of their increased stability to sintering under the action of the reaction medium at elevated temperatures (up to 300-350 ° C).
According to the invention, other neutral or weakly acidic carriers, such as silica or alumina, can be used as carriers. The use of acid carriers such as aluminosilicates (zeolites) as carriers is limited, since under the conditions of the hydrogenation / dehydrogenation reactions, the side reactions of cracking and ring opening that lead to the irreversibility of the stages are possible.
A catalytic composite material for storing hydrogen may contain both a structure-forming modifier or a matrix in which the source of hydrogen (organic matter) and the catalyst are dispersed. As such a matrix-forming matrix, for example, a block catalyst (for example, based on corderite) with an active metal (platinum) applied can be used, a source of hydrogen (for example, hydrogenated biphenyl) is distributed in the pores of this block.
According to the invention, organic compounds (aromatic hydrocarbons, including condensed and polycyclic, polyunsaturated and aromatic oligomers and polymers, for example polyacetylenes, polycubulins) capable of reversibly and repeatedly hydrogenating-dehydrogenated are used as hydrogenation (substrate) in the stage of refueling the catalyst system with hydrogen. . Preferably, the use of terphenyl, for example as a mixture of ortho-, meta-, and para-isomers.
As a substrate, instead of terphenyl, biphenyl, naphthalene, anthracene, and low molecular weight polymers of the polystyrene type with an average molecular weight of up to 1000 can be used, although it is possible to use higher molecular substrates.
The choice of such hydrocarbons as a substrate for molecules is due to the fact that under the hydrogenation conditions of these compounds and the dehydrogenation of their saturated derivatives, these substances are in a liquid state and have low volatility (low saturated vapor pressure), which contributes to the formation of a stable suspension of catalyst particles in the catalyst system and It promotes the flow of catalytic reactions with high velocities.
The physico-chemical properties of terphenyl isomers are listed below.
O-terphenyl: m.p. = 58-59 ° C; T. B. = 337 ° C
M-terphenyl: m.p. = 86-87 ° C; T. B. = 379 ° C
P-terphenyl: m.p. = 212-213 ° C; T. B. = 389 ° C.
Obviously, under the conditions of the process, the reaction mixture of terphenyl and hydrogenated product will be in the liquid state, and its entrainment with the gas stream (hydrogen) can be reduced to zero.
Limitations in the use of biphenyl are due to the fact that both biphenyl itself and its hydrogenated derivative (bicyclohexyl) have boiling points (biphenyl bicarbonate, 255 ° C) in the range of the temperature requirements for the second stage of the proposed method (hydrogen evolution during Dehydrogenation, see below), which can lead to partial entrainment of the substrate with hydrogen flow.
The proposed method for storing hydrogen in catalytic systems consists of two stages:
1. the steps of charging the system with hydrogen during contacting the starting substrate (e.g., terphenyl) and a heterogeneous catalyst containing a highly dispersed metal, for example platinum, in a metal heated vessel (autoclave), preferably but not necessarily equipped with a device for stirring the catalyst system 500 rpm (mechanical stirrer with a hydraulic seal), at temperatures in the range of 80-180 ° C, preferably 100-150 ° C, a hydrogen pressure of 5-100 atm, preferably 5-20 atm, and a substrate: catalyst ratio of 10: 1 -1000: 1, preferably from 20: 1 to 100: 1.
2. The steps of extracting hydrogen from the system by contacting a substrate already pre-hydrogenated in the first stage, for example terphenyl, with the same catalyst at temperatures of 220-340 ° C, preferably 270-320 ° C and atmospheric pressure.
The amount of hydrogen that the catalytic system can accumulate by the method of the invention can reach 7.5-8.0% by weight at the maximum substrate: catalyst ratio. This capacity value includes hydrogen introduced into the system during the complete hydrogenation of the substrate, for example a polycyclic aromatic hydrocarbon or oligomer, dissolved hydrogen and hydrogen adsorbed on the catalytic center (metal particles of nickel, platinum or palladium).
The observed technical effects-the multiple refilling of the catalytic systems proposed in the present invention by hydrogen, the high rate of the hydrogenation and dehydrogenation stages are explained by the fact that the catalysts containing platinum are highly active and selective in the hydrogenation / dehydrogenation reactions, and highly stable to high temperatures. The use of a heterogeneous catalyst allows, if necessary (recharging the system with a new catalyst), to separate the catalyst for its regeneration. The use of high-boiling substrates avoids the need for additional devices for separating hydrogen from the volatile substance of the catalytic system.
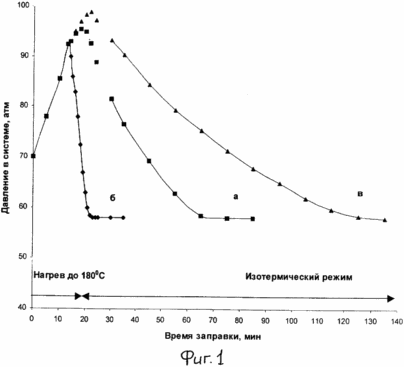 |
FIG. 1 shows pressure curves in the system (autoclave) versus the time of hydrogen charging at its initial pressure of 70 atm for catalytic systems: A - 5% Pt / sibunit - terphenyl B - 15% Pt / C - terphenyl In - 0,7% Pd / sibunit - terphenyl. |
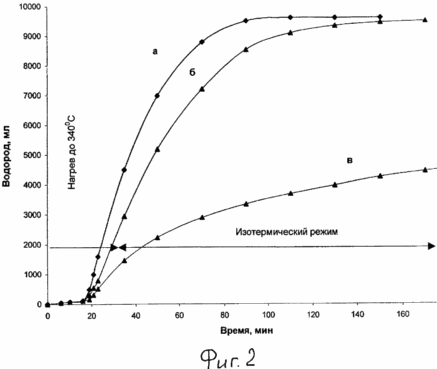 |
2 shows the curves of the dependence of the amount of released hydrogen on the time of reduction of the dehydrogenation step for catalytic systems: A - 15% Pt / C - terphenyl B - 5% Pt / sibunite - terphenyl In - 0,7% Pd / sibunit - terphenyl. |
The invention is illustrated by the following examples:
Example 1 .
2.9 g of a 2% Pd / CKT-4 catalyst (activated carbon) are impregnated in a liquid capacity with molten biphenyl (3.5 g) in a glass container, the resulting catalytic system (total weight 6.6 g) is placed along with the capacity in a PARR- 4842 (300 ml, equipped with pressure gauges), the autoclave is blown with hydrogen (this operation is lowered when the system is reused) and heated to 120 ° C for 1 hour at a pressure of 50 atm. After cooling to room temperature, unreacted hydrogen is vented from the system and the system is heated to 300 ° C with the measurement of the amount of released hydrogen with a gas clock. The amount of hydrogen that can theoretically be spent on hydrogenation of all loaded biphenyl is 3.05 liters. The total amount of hydrogen released from the catalytic system during its heating (minus the gas volume due to its thermal expansion) was 3.3 liters (or 0.295 g).
Thus, the proposed system is capable of storing 4.6% by weight of hydrogen. Note that the loss of mass of the catalytic system (determined by weighing) after one cycle, filling / discharging does not exceed 0.5%, which indicates an insignificant entrainment of the substrate with the gas flow.
Example 2 .
0.6 g of 5% Pt / Sibunit catalyst (graphitized activated carbon) and 11 g of terphenyl according to example 1 are charged to the autoclave and 11 g of terphenyl according to Example 1 and at a pressure of 70 atm and stirred at 500 rpm, the mixture is heated to 180 ° C for 1.5 hours . The amount of hydrogen that can theoretically be spent on hydrogenation of all loaded terphenyl is 9.55 liters. The kinetic curve of hydrogen absorption during hydrogenation of terphenyl, based on the readings of the manometer, is shown in Fig. 1 (a).
Next, according to Example 1, the total amount of hydrogen released from the catalyst system during its heating to 340 ° C is determined. It was 9.75 liters (or 0.87 g). The kinetic curve of hydrogen evolution is shown in Fig. 2 (b).
Thus, the proposed catalyst system with a total weight of 11.6 g is capable of accumulating 7.5% by weight of hydrogen.
Example 3 .
The catalyst system with the difference that 15% Pt / C was used as the catalyst was tested in the hydrogen storage method of Example 2. Kinetic hydrogen absorption curves during hydrogenation of the terphenyl and hydrogen evolution in the dehydrogenation step are shown in FIG. 1 (b) and 2 (a). The total amount of hydrogen released from the catalyst system during its heating to 340 ° C, and as in Example 2, was 9.75 liters, i.e. The system is able to accumulate 7.5% by weight of hydrogen.
It should be noted that when the catalyst of Example 3 is used, it is essential (in comparison with Example 2) that the charging times and the complete hydrogen evolution in the dehydrogenation step are reduced, which is obviously associated with different catalyst activities. Thus, the complete hydrogenation of terphenyl [is observed, the cessation of hydrogen absorption, see Fig. 1 (b)] is achieved already by the 22nd minute, and the hydrogen evolution ends up to 90 minutes [Fig. 2 (a)].
The catalytic system was tested in a hydrogen storage method in 4 cycles of hydrogenation / dehydrogenation. The total capacity of the system for hydrogen in 4 cycles is reduced by no more than 1% (assuming a maximum hydrogen capacity of 100%), while there is no decrease in the rates of hydrogenation and dehydrogenation. Some decrease in the total capacity is associated with the loss from the system in the first cycles of more readily boiling hydrocarbons (impurity biphenyl - about 1%).
Examples 4-7 .
The catalyst system of Example 3 was tested in a hydrogen storage method with the difference that other temperatures, hydrogen initial pressures, and stirring rates were used in the hydrogenation / dehydrogenation steps.
The test results are shown in Table 1.
It can be seen that at a constant capacity of the system with respect to hydrogen, depending on the temperature and the initial pressure of hydrogen in the autoclave, the times of the hydrogenation / dehydrogenation steps vary substantially.
It is seen from Example 5 that the charging of the catalyst system with hydrogen during the hydrogenation of terphenyl and the evolution of hydrogen as a result of dehydrogenation are possible even without its mechanical mixing. Obviously, this is very important for mobile and portable hydrogen storage devices, in which mixing is difficult to organize.
Examples 6 and 7 show that charging of the system is possible at lower hydrogen pressures (even 5 atm and lower).
Table 2 shows the results of testing various catalytic systems according to the invention.
| Table 1 Test results of the catalytic system 15% Pt / C: terphenyl = 1: 18.4 (wt.) | |||||||||
| N App. | The conditions of the hydrogenation step | Time of 50% / 100% refueling, min | The conditions of the dehydrogenation step | Time of 50% / 100% release of hydrogen, min | Hydrogen capacity, mass% | ||||
| Р, atm | T, ° C | N, rpm | T, ° C | N, rpm | |||||
| 3 | 70 | 180 | 500 | 15/22 | 340 | 500 | 38/90 | 7.5 | |
| 4 | 50 | 140 | 300 | 28/60 | 280 | 300 | 50/320 | 7.5 | |
| 5 | 50 | 140 | 0 | 50/400 | 280 | 0 | 130/0 | 7.5 | |
| 6th | 5 (const) | 180 | 500 | 50/180 | 7.5 | ||||
| 7th | thirty | 180 | 500 | 26/100 | |||||
| table 2 | ||||||
| Test results for various catalytic systems | ||||||
| The conditions for carrying out the hydrogenation step: P = 20 atm, T = 180 ° C, N = 500 rpm | ||||||
| The conditions for carrying out the dehydrogenation step: P = 1 atm, T = 320 ° C, N = 500 rpm | ||||||
| N App. | Catalyst | Substrate | Substrate / Catalyst, wt. | Time of 50% / 100% refueling, min | Time of 50% / 100% release of hydrogen, min | Hydrogen capacity, mass% |
| 8 | 15% Pt / C | Terpene | 2/1 | 10/17 | 25/60 | 4.2 |
| 9 | " | 100/1 | 80/210 | 100/320 | 7.6 | |
| 10 | " | 800/1 | 380/900 | 400 / - | 7.9 | |
| eleven | 3% Pt-0.25% Pd / | Terpene | 20/1 | 25/70 | 70/240 | 7.5 |
| 12 | "Sibunit | 500/1 | 340/850 | 520 / - | 7.7 | |
| 13 | 5% Pd / Al 2 O 3 | naphthalene | 20/1 | 20/60 | 55/140 * | 6.2 |
| 14 | " | 100/1 | 90/220 | 6.3 | ||
| 15 | 15% Pt / C | Polystyrene | 20/1 | 30/120 | 60/180 | 5.7 |
| 16 | " | M в = 1000 | 100/1 | 110/260 | 240 / - | 5.8 |
| 17th | 15% Ni / SiO 2 | Polystyrene | 5/1 | 50/160 | 140/400 | 4.8 |
| 18 | " | M в = 1000 | 40/1 | 200/640 | 5.7 | |
| * - in the dehydrogenation stage, when using naphthalene, a hydrogen permeable membrane is provided as a substrate at the outlet of the autoclave to prevent decalin from escaping with hydrogen (hydrogenation product of naphthalene) | ||||||
CLAIM
1. A catalytic composite material for storing hydrogen containing as an hydrogen source an organic substrate capable of reversible hydrogenation and dehydrogenation and a hydrogenation-dehydrogenation catalyst, characterized in that, as an organic substrate, it comprises an aromatic hydrocarbon selected from the group consisting of condensed, Polycyclic, polyunsaturated, aromatic oligomers and polymers, and as a catalyst the material comprises a heterogeneous catalyst comprising a carbon or oxide carrier with a high specific surface area deposited on the surface with at least one Group VIII metal at a substrate to catalyst ratio of 10: 1 to 1000: 1.
2. The material of claim 1, wherein the polycyclic aromatic hydrocarbon comprises biphenyl or a functional derivative thereof or terphenyl.
3. Материал по п.1, отличающийся тем, что в качестве конденсированного ароматического углеводорода содержит нафталин, или антрацен, или функциональное производное того или другого.
4. Материал по п.1, отличающийся тем, что в качестве ароматического полимера содержит полистирол или его сополимер со средним молекулярным весом до 1000, или полиацетилен, или поликумулен.
5. Материал по п.1, отличающийся тем, что в качестве углеродного носителя содержит активированный уголь.
6. Материал по п.1, отличающийся тем, что в качестве оксидного носителя содержит оксид кремния или оксид алюминия.
7. Материал по п.1, отличающийся тем, что в качестве металла VIII группы содержит платину, или палладий, или никель, или сплав платины с палладием в количестве от 0,1 до 15% от массы гетерогенного катализатора.
8. Способ хранения водорода путем заправки водородом при повышенном давлении каталитического композитного материала, содержащего в качестве источника водорода органический субстрат, способный к обратимой реакции гидрирования-дегидрирования, и катализатор гидрирования-дегидрирования, и выделения водорода из каталитического композитного материала при его нагреве при пониженном давлении, отличающийся тем, что в качестве каталитического композитного материала используют материал по любому из пп.1-7, заправку его осуществляют при контактировании органического субстрата и гетерогенного катализатора при температуре от 50 до 180°С и давлении водорода от 1 до 100 атм, а выделение водорода осуществляют при контактировании гидрированного при заправке органического субстрата с тем же катализатором при температуре от 200 до 350°С при атмосферном давлении.
9. Способ по п.8, отличающийся тем, что заправку и выделение водорода осуществляют без перемешивания композитного материала.
print version
Date of publication 28.02.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.