| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2285859
![]()
CAPACITY FOR STORAGE AND ACCUMULATION OF HYDROGEN
The name of the inventor: Chabak Alexander Fedorovich (RU)
The name of the patent holder: Chabak Alexander Fedorovich (RU)
Address for correspondence: 123585, Moscow, ul. Berzarina, 19, building 1, ap. 203, A.F. Chabaku
Date of commencement of the patent: 2005.03.29
The invention relates to the field of hydrogen energy - accumulation and storage of hydrogen, which is currently used in chemical, transport engineering and other industries. A container for storing and storing hydrogen consists of a sealed enclosure, process connections, a heater and a hydrogen storage accumulator disposed in the housing, the container being divided by a proton-conductive material partition into an anodic cavity filled with water with a porous anode disposed therein and a cathode cavity With a continuous cathode and a heater located in it and filled with hydrogen storage-accumulator, which is a microporous structure made of high-strength material. In this case the barrier is made in the form of a proton-conducting membrane. The microporous structure is made of hollow microspheres. In addition, the microporous structure is made of polymers of the aramid group. And the microporous structure can be made of a foam metal, for example, foam-nickel, penotitanium. In addition, the microporous structure is made of a material with proton-conducting properties. The invention provides the creation of a container for the safe storage and storage of hydrogen with an increase in the mass content of hydrogen above 6% .
DESCRIPTION OF THE INVENTION
The invention relates to the field of hydrogen energy - accumulation and storage of hydrogen, which is currently used in chemical, transport engineering and other industries.
Hydrogen accumulation and storage devices based on hydrogen binding in a solid material (for example, in metal hydrides or sorption on the surface of dispersed nanomaterials) are known ( RF patents No. 2037737, 2038525, IPC F 17 C 5/04 ), these storage devices And hydrogen storage are the most explosion-proof of existing ones. Hydrogen does not have excess pressure, but such systems are inertial and require a certain time (on the order of several minutes) to start work, absorption and evolution of hydrogen occurs with significant thermal effects, in addition, the mass content of hydrogen - the ratio of the weight of hydrogen contained in the battery to the weight of the battery itself - 4.5% - is very low. The mass content depends both on the amount of hydrogen in the accumulating material and on the specific gravity of the accumulating material.
A storage tank for hydrogen is known ( patent No. 2222749, IPC F 17 C 5/04 ), which is a sealed housing with an internal container for storing liquefied hydrogen, the gas filling system being designed to reduce hydrogen losses and reduce tank charging time. This tank is designed for a hydrogen vehicle ( Schwarz A. The car of the future, J. Vestnik, No. 10 (347), p.1-5, 12.05.2004 ), it is made of strong composite relatively light materials. The latest version has a volume of 90 liters, weight 40 kg , hydrogen pressure 400 atm . Estimates show that in this case 3.2 kg of hydrogen can be stored in the container, therefore, the mass content of hydrogen is 3.2 / 40 × 100% = 8% . Disadvantages of the capacity is the explosion hazard and low hydrogen content per unit volume, up to 400 liters of hydrogen per liter , loss of gas from the tank.
It is known that it is possible to store hydrogen in hollow microspheres made of glass with a diameter of 5-200 μm and a wall thickness of 0.5-5 μm ( Malyshenko SP, Nazarova OV Hydrogen accumulation., In the collection of articles. "Atomic-hydrogen Energy and technology ", issue 8, pp.155-205, 1988 ). At a temperature of 200-400 ° C under pressure, hydrogen, actively diffusing through the walls, fills the microspheres and, after cooling, remains in them under pressure. Thus, at a hydrogen pressure of 500 atm and heating the microspheres to the indicated temperatures, a mass content of hydrogen in the microspheres of 5.5-6.0 % was obtained. At a lower pressure, the mass content of hydrogen in the microspheres will decrease. When heated to 200 ° C, about 55% of the hydrogen stored in microspheres and about 75% are released when heated to 250 ° C. When hydrogen is stored in glass microspheres, the diffusion through the walls is about 0.5% per day. In the case of coating microspheres with metallic films, the diffusion loss of hydrogen at room temperature decreases by a factor of 10-100 . A significant drawback is that charging the battery with microspheres is carried out at relatively low hydrogen pressures, since the tensile strength of the glass has low values and is within the limits of up to 20 kg / mm 2 . This makes it impossible to provide a mass content of hydrogen in microspheres, significantly exceeding 6 wt.% .
A container for storing and accumulating hydrogen is known, consisting of a sealed enclosure, process connections, an internal heat exchange surface and a hydrogen storage vehicle, which is a powder of an intermetallic compound ( RF patent No. 2037737, IPC F 17 C 5/04 - prototype ). A disadvantage of the invention is that the absorption and evolution of hydrogen occurs with significant thermal effects, in addition, the mass content of hydrogen - the ratio of the weight of hydrogen contained in the container to the weight of the container itself - 4.5% - is very low.
The technical result, to which the invention is directed, is the creation of a container for the safe storage and accumulation of hydrogen, providing an increase in the mass content of hydrogen above 6% .
To this end, a hydrogen storage and storage container is proposed, consisting of a sealed housing, process connections, a heater and a hydrogen storage accumulator disposed in the housing, wherein the container is divided by a proton-conducting material partition into an anode cavity filled with water, with a porous anode disposed therein , And a cathodic cavity with a continuous cathode and heater disposed therein and filled with a hydrogen storage accumulator made of a material with a tensile strength above 30 kg / mm2 and having a microporous structure.
In this case the barrier is made in the form of a proton-conducting membrane.
The microporous structure is made of hollow microspheres.
In addition, the microporous structure is made of polymers of the aramid group. And the microporous structure can be made of a foam metal, for example, foam-nickel, penotitanium.
In addition, the microporous structure is made of a material with proton-conducting properties.
The hydrogen content in the microporous structure is determined primarily by the strength characteristics of the material of this structure. For microporous structures for tanks for storage and storage of hydrogen, high-strength materials with a tensile strength ![]() Higher than 30 kg / mm 2 . From the strength characteristics, it depends which maximum hydrogen pressure can be created at a fixed pore size, since the same hydrogen pressure generates large stresses in pores of large sizes and accordingly a lower voltage in small pores. By increasing the pore volume (and hence their size), we obtain a larger hydrogen content per unit volume of the microporous structure, but the increase in the pore size is limited by the magnitude of the limiting stresses that are created by the hydrogen pressure in these pores. As a result, for each material, the maximum maximum pore size is determined by the strength characteristics of the material of the microporous structure. In addition, the material of the microporous structure should have substantially different characteristics for hydrogen permeability under various conditions, for example, with temperature changes, ultrasound, high frequency currents, constant or alternating voltage, and the like. The nature of the impact and its magnitude are determined by the requirements of the rate of hydrogen absorption by the microporous structure and / or the rate of hydrogen release from it.
Higher than 30 kg / mm 2 . From the strength characteristics, it depends which maximum hydrogen pressure can be created at a fixed pore size, since the same hydrogen pressure generates large stresses in pores of large sizes and accordingly a lower voltage in small pores. By increasing the pore volume (and hence their size), we obtain a larger hydrogen content per unit volume of the microporous structure, but the increase in the pore size is limited by the magnitude of the limiting stresses that are created by the hydrogen pressure in these pores. As a result, for each material, the maximum maximum pore size is determined by the strength characteristics of the material of the microporous structure. In addition, the material of the microporous structure should have substantially different characteristics for hydrogen permeability under various conditions, for example, with temperature changes, ultrasound, high frequency currents, constant or alternating voltage, and the like. The nature of the impact and its magnitude are determined by the requirements of the rate of hydrogen absorption by the microporous structure and / or the rate of hydrogen release from it.
The simplest and really created microporous structure is a structure created from hollow microspheres, primarily metals or their alloys, and a microporous structure made from foam-nickel, penotitanium, other foam metals and from polymeric materials.
A microporous structure of hollow microspheres, for example of steel, is formed into a single rigid structure. This can be done by diffusion welding. In this case, all the free space both inside the microspheres and between them will be filled with hydrogen.
Of exceptional interest for the creation of a porous microstructure are materials having high strength characteristics and low specific gravity, these are primarily composite carbon and polymer materials. Thus, polymers made on the basis of poly-p-phenylene terephthalamide and other similar polymers of aromatic series (aramids) have a specific gravity of 5.5 times less than steel, and strength characteristics are 2.5-3.5 times higher. For high strength steels, the tensile strength ![]() = 160-220 kg / mm 2 , for aramids tensile strength up to 550 kg / mm 2 ( Table 1 ).
= 160-220 kg / mm 2 , for aramids tensile strength up to 550 kg / mm 2 ( Table 1 ).
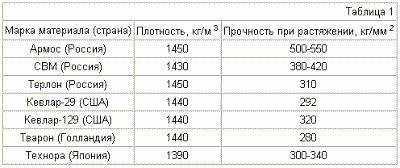
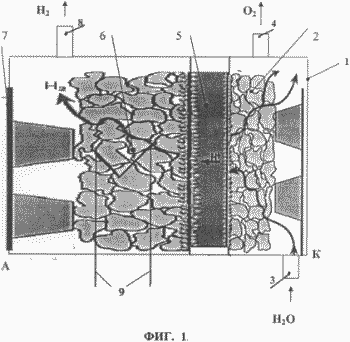
FIG. 1 is a schematic diagram of a container for storing and storing hydrogen, where 1 is the vessel body, 2 is a porous electrode, an anode made of a first kind conductor, 3 is a branch pipe for supplying water to the anode cavity, 4 is a branch pipe for removing oxygen from Anode cavity, 5 - a proton-conducting material membrane (membrane), 6 - a microporous structure - a hydrogen accumulator, 7 - a solid electrode - a cathode made of a conductor of the first kind, 8 - a branch pipe for hydrogen removal from the tank to the engine (consumer), 9 Heater.
 |
 |
2 shows a microporous structure of microspheres, where 10 is a microsphere.
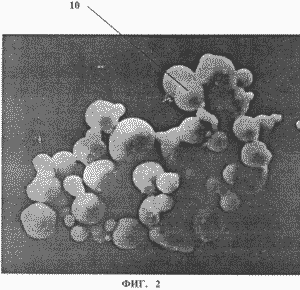
3 shows a microporous structure of polymeric material - armos, where 11 - fibers, 12 - pores.
DEVICE WORKS AS FOLLOWING
The sealed container body 1 is divided by a partition 5 into two cavities. The anode cavity is filled with water through the nozzle 3. Water enters the porous anode 2. At the boundary of the porous anode made, for example, from porous titanium and the proton-conducting membrane 5, which can be made of ceramic, polymer or other material, the oxidation of water proceeds:
2H 2 O + 2e - = O 2 + 4H + .
Oxygen through the pores of the anode is released into the volume of water and through the branch pipe of oxygen removal 4 is removed. Hydrogen ions (protons) along the proton-conducting membrane 5 move to the cathode 7, where they are reduced to hydrogen. The hydrogen does not pass through the solid metal cathode 7 and saturates the microporous structure 6. The cathode and the proton-conducting membrane form a cathode cavity filled with a porous microstructure 6. From this closed volume, hydrogen, when heated by the heater 9 through the pipe 8, is directed to the consumer, for example, to the hydrogen supply system to the engine Internal combustion or fuel cells. To accelerate saturation with hydrogen, the microporous structure can have proton-conducting properties. The amount of hydrogen in the porous structure is determined by the magnitude of the charging current and charging time.
Let's compare the characteristics of a container for storing and accumulating hydrogen with a microporous structure of hollow microspheres 10 ( see Fig. 2 ) made of steel and armos (see Fig. 3), where 11 are the fibers of the material. With the formation of pores 12, their shape can be the most diverse from capillaries to spheres. Consider a variant of spherical pores.
Table 2 compares the characteristics of microporous structures made of steel microspheres and a microporous structure with the same pore size made of armos. In the tables - ![]() - tangential stress on the microsphere shell, kg / mm 2 ,
- tangential stress on the microsphere shell, kg / mm 2 , ![]() - radial stress on the microsphere shell, kg / mm 2 . The specific gravity of steel is 8 kg / l.
The specific weight of armos is 1.45 kg / l .
- radial stress on the microsphere shell, kg / mm 2 . The specific gravity of steel is 8 kg / l.
The specific weight of armos is 1.45 kg / l .
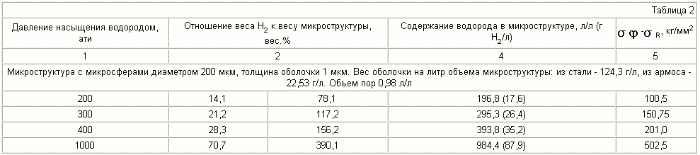
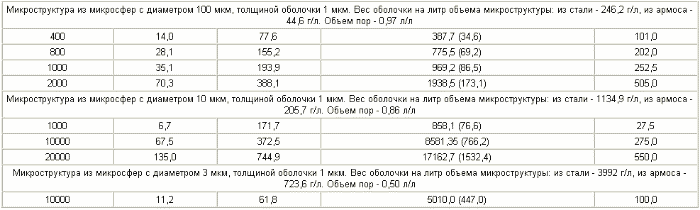
As can be seen from Table 2 , for the same microporous structures with micropores with a diameter of 200 μm, the mass content of hydrogen in the microstructure is reached for the best steels of 28.3 wt% , and for the reinforcement 390 wt% .
Example 1
The storage tank for hydrogen is separated by a high-temperature (up to 300 ° C ) proton-conducting ceramic membrane into two cavities. The cathode cavity with a volume of 0.028 liters is filled with a microporous structure of hollow microspheres of high-strength steel with a diameter of microspheres of 200 and 80 microns , a shell thickness of 1 μm . Microspheres are connected in a rigid nozzle by diffusion welding. Weight 0.028 liters of microporous structure - 3.5 g. The anode of porous titanium is washed with water. Charging of the microporous structure was carried out at a current density of 1 A / cm 2 . Within 20 minutes, 7.2 liters of hydrogen passed through the surface of the proton-conducting membrane into the volume of the microporous structure. When charging, the temperature of the microporous structure was maintained by a special heater at 280 ° C.
The mass content of hydrogen was 18.4 wt.% .
Example 2
The same device was loaded with a microporous structure based on polymer-armos, which is a polymer fiber with a pore size of ~200 μm . Charging the microporous structure with hydrogen was also carried out for 20 minutes . Proton conducting polymer membrane - MF-4SK . The microporous structure absorbed 7.2 liters of hydrogen. The weight of the porous structure was 0.64 g . The mass content of hydrogen in the microstructure is 101% .
Such storage and storage tanks for hydrogen have significant advantages over those that are charged with hydrogen at high pressure or with the help of cryogenic technologies. They are not only safe, but have a very high degree of hydrogen saturation while maintaining small dimensions. They can be supplied not only by fuel stations or special delivery points of batteries, these can be charged by consumers (motorists) themselves, for this purpose it is enough to pour clean water into the cavity with the anode and connect the capacitor to the mains (power supply).
CLAIM
1. A hydrogen storage container consisting of a sealed housing, process connections, a heater and a hydrogen storage accumulator disposed in the housing, characterized in that the container is divided by a proton-conducting material partition into an anode cavity filled with water, with a porous anode located therein, And a cathodic cavity with a continuous cathode and heater disposed therein, and filled with a hydrogen storage accumulator made of a material with a tensile strength above 30 kg / mm2 and having a microporous structure.
2. A container according to claim 1, characterized in that the filler-battery is made of hollow microspheres.
3. A container according to claim 1, characterized in that the filler-battery is made of polymers of the aramid group.
4. A container according to claim 1, characterized in that the filler-battery is made of a foam metal, for example, foam-nickel, penotitanium.
5. The container according to claim 1, characterized in that the partition is in the form of a proton-conducting membrane.
6. A container according to claim 1, characterized in that the filler-battery is made of a material with proton-conducting properties.
print version
Date of publication 31.10.2006гг




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.