| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2283453
![]()
CAPACITY FOR STORAGE OF HYDROGEN AND METHOD OF HYDROGEN BATTERY
The name of the inventor: Chabak Alexander Fedorovich (RU)
The name of the patent holder: Chabak Alexander Fedorovich (RU)
Address for correspondence: 123585, Moscow, ul. Berzarina, 19, building 1, ap. 203, A.F. Chabaku
Date of commencement of the patent: 2006.04.10
The invention relates to the field of hydrogen energy, storage and storage of hydrogen, used in chemical, transport engineering and other industries. To reduce pressure and temperature in the stages of accumulation and storage of hydrogen, increase the mass content of hydrogen, reduce hydrogen losses during storage and accumulation in a hydrogen storage tank consisting of a sealed enclosure, process connections, an internal heat exchange surface and a hydrogen storage accumulator housed in a housing , Hydrogen filler-accumulator is hollow microspheres made of highly conductive material. The method for hydrogen storage is to saturate the microspheres with hydrogen by diffusion, while placing microspheres that are a cathode into a hydrogen-containing medium, and saturation of the microspheres is carried out with hydrogen converted into an ionic form. The transfer of hydrogen into the ionic form can be carried out by electrolysis in aqueous solutions. The transfer of hydrogen into the ionic form can be carried out by ionization, for example, in an electric discharge.
DESCRIPTION OF THE INVENTION
The invention relates to the field of hydrogen energy - accumulation and storage of hydrogen, which is currently used in chemical, transport engineering and other industries.
Hydrogen accumulation devices and hydrogen accumulation methods based on hydrogen binding in a solid material (for example, in metal hydrides or sorption on the surface of dispersed nanomaterials) are known ( RF patents Nos. 2,037,737, 2,038,525 of the MPC F 17 C 5/04 ), these methods And devices for accumulation and storage of hydrogen are the most explosion-proof of the existing ones. Hydrogen does not have excess pressure, but such systems are inertial and require a certain time (on the order of several minutes) to start work, absorption and evolution of hydrogen occurs with significant thermal effects, in addition, the mass content of hydrogen - the ratio of the weight of hydrogen contained in the battery to the weight of the Battery - 4.5%, is very low. The mass content depends both on the amount of hydrogen in the accumulating material and on the specific gravity of the accumulating material.
A storage tank for hydrogen is known (patent No. 2222749 of the IPC F 17 C 5/04) , which is a sealed housing with an internal vessel for storing liquefied hydrogen, the gas filling system being designed to reduce hydrogen losses and reduce tank charging time. This tank is designed for a hydrogen vehicle ( Schwarz A. The car of the future, J. Vestnik, No. 10 (347), p.1-5, 12.05.2004 ), it is made of strong composite relatively light materials. The last modification has a volume of 90 liters , a mass of 40 kg , a hydrogen pressure of 400 atm . Estimates show that in this case 3.2 kg of hydrogen can be stored in the tank, therefore, the mass content of hydrogen is 3.2 / 40 · 100% = 8% . Disadvantages of the capacitance are explosive and low hydrogen content per unit volume, up to 400 liters of hydrogen per liter , loss of gas from the tank.
A hydrogen storage container is known , consisting of a sealed enclosure, process connections, an internal heat exchange surface, and hydrogen filler, which is a powder of an intermetallic compound ( RF patent No. 2037737, IPC F 17 C 5/04 - prototype ). Disadvantages of the invention are that the absorption and evolution of hydrogen occurs with significant thermal effects, in addition, the mass content of hydrogen - the ratio of the weight of hydrogen contained in the container to the weight of the container itself - 4.5% , is very low.
Known is the way of accumulating hydrogen in microspheres (Malyshenko SP Nazarova OV Hydrogen accumulation: in the collection of articles "Atomic-hydrogen energy and technology", issue 8, pp. 155-205. 1988 - prototype) . Hollow microspheres are made of glass with a diameter of 5-200 microns with a wall thickness of 0.5-5 microns . At a temperature of 200-400 ° C under pressure, hydrogen, actively diffusing through the walls, fills the microspheres and, after cooling, remains in them under pressure. Thus, at a hydrogen pressure of 500 atm and heating of the microspheres to the indicated temperatures, a mass content of hydrogen in microspheres of 5.5-6.0 % was obtained. At a lower pressure, the mass content of hydrogen in the microspheres will decrease. When heated to 200 ° C, about 55% of the hydrogen stored in microspheres and about 75% are released when heated to 250 ° C. When hydrogen is stored in glass microspheres, the diffusion through the walls is about 0.5% per day. In the case of coating microspheres with metallic films, the diffusion loss of hydrogen at room temperature decreases by a factor of 10-100 . A significant disadvantage of this method of hydrogen storage is that the charging of the battery with microspheres occurs at high hydrogen pressures and at elevated temperatures, which leads to an increased danger of the process.
The technical result to which the invention is directed is the reduction of pressure and temperature in the stages of accumulation and storage of hydrogen, increase of the mass content of hydrogen, reduction of hydrogen losses during storage and accumulation, which will lead to an increase in safety and economy.
For this purpose, a storage tank for hydrogen and a method for hydrogen storage have been proposed.
The hydrogen storage container consists of a sealed enclosure, process connections, an internal heat exchange surface and a hydrogen storage tank located in the housing, wherein the hydrogen filler-accumulator is hollow microspheres of a conductive material with a tensile strength of more than 30 kg / mm 2 .
In this case, steel or titanium, or lanthanum, or nickel, or zirconium, or alloys based on these metals or graphite, or compositions based on graphite are used as the microsphere material.
The diameter of the microspheres can decrease from the center of the body to the periphery.
Each microsphere may have a coating of a metal absorbing hydrogen, for example palladium or nickel, or an alloy of lanthanum with nickel.
The vessel body can be made of a non-conductive material with a negative electrode disposed inside and a branch pipe for supplying a hydrogen-containing medium, the positive electrode being located outside the housing.
The proposed method of hydrogen storage is to saturate the microspheres with hydrogen by diffusion, while placing microspheres, which are a negative electrode, into the hydrogen-containing medium, and saturation of the microspheres is carried out with hydrogen converted into an ionic form.
The transfer of hydrogen into the ionic form is carried out by electrolysis in aqueous solutions.
The transfer of hydrogen into the ionic form is carried out by ionization, for example, in an electric discharge.
 |
 |
 |
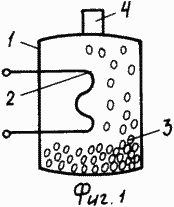
1 shows a general view of a hydrogen storage tank, where 1 is a housing, 2 is a heat exchange surface, 3 is a microsphere, and 4 is a process connection.
In this embodiment, the body 1 can be made of any material, the microspheres 3 fill the entire body and, for hydrogen storage, they are discharged from the housing. The discharged microspheres to which a negative potential is applied are placed, for example, in an electrolyte solution (aqueous solution of sulfuric acid, hydrazine hydrate, etc.), to accelerate the diffusion of hydrogen through the shell of the microspheres, hydrogen is transferred to the ionic form during electrolysis and saturates the internal cavity of the microspheres Hydrogen.
Microspheres can be saturated with hydrogen and during ionization of hydrogen in an electrical discharge. The microspheres are also a cathode ( see Example 4 ).
Microspheres filled with hydrogen are again loaded into the body 1 and when heated by the heat exchange surface 2 of them hydrogen will be released, supplied to the consumer through the process connection 4.
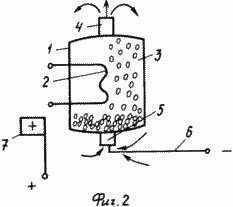
FIG. 2 shows a general view of a hydrogen storage tank in which the microspheres are saturated with hydrogen directly in the vessel, the body 1 being made of a non-conductive material and having an additional branch pipe 5 for supplying a hydrogen-containing medium that opens only during saturation of the microspheres with hydrogen, The negative electrode 6 is placed in the housing and the positive electrode 7 is located outside the housing 1. In this case, the container is placed in a hydrogen-containing medium, the branch pipe 5 is opened, the corresponding potentials are applied to the electrodes 6 and 7, and hydrogen is accumulated without unloading the microspheres from the vessel during electrolysis or In an electrical discharge.
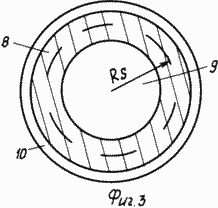
3 shows a microsphere, where 8 is a shell, 9 is a cavity filled with hydrogen, 10 is a metal coating.
Real materials - high-strength (with ![]() Vr is the strength limit of more than 30 kg / mm 2 ), conductive, from which it is possible to make microspheres with a diameter of 1 to 50 μm with a wall thickness of ~ 1 μm, are steel or titanium, or lanthanum, or nickel, or zirconium, or alloys on Based on these metals or graphite, or compositions based on graphite. In this case, the saturation of microspheres with hydrogen can be intensified by covering them with a layer of metal 13 ~ 0.1 μm thick with a high ability to absorb hydrogen, for example, palladium or nickel or an alloy of lanthanum with nickel.
Vr is the strength limit of more than 30 kg / mm 2 ), conductive, from which it is possible to make microspheres with a diameter of 1 to 50 μm with a wall thickness of ~ 1 μm, are steel or titanium, or lanthanum, or nickel, or zirconium, or alloys on Based on these metals or graphite, or compositions based on graphite. In this case, the saturation of microspheres with hydrogen can be intensified by covering them with a layer of metal 13 ~ 0.1 μm thick with a high ability to absorb hydrogen, for example, palladium or nickel or an alloy of lanthanum with nickel.
In such microspheres, a hydrogen pressure of several thousand atmospheres can be created. For example, a microsphere with a diameter of 10 μm with a shell thickness of 1 μm , made of steel 30Х (
![]() 0.2 = 75 kg / mm 2 ,
0.2 = 75 kg / mm 2 ,
![]() Vp = 90 kg / mm 2 ) , can withstand a pressure of 3000 atm .
Vp = 90 kg / mm 2 ) , can withstand a pressure of 3000 atm .
Where ![]() 0.2 - yield strength, kg / mm 2 ,
0.2 - yield strength, kg / mm 2 ,
![]() Вр - ultimate strength, kg / mm 2 .
Вр - ultimate strength, kg / mm 2 .
As
![]()
![]() = PR s / 2S, a
= PR s / 2S, a
![]() R = P / 2,
R = P / 2,
Where ![]()
![]() - tangential stress on the microsphere shell, kg / mm 2
- tangential stress on the microsphere shell, kg / mm 2
P is the hydrogen pressure in the microsphere, kg / mm 2 ,
R S is the radius of the microsphere to the middle of the shell thickness, mm ,
S - shell thickness, mm
![]() R - radial stress on the microsphere shell, kg / mm 2
R - radial stress on the microsphere shell, kg / mm 2
Then ![]()
![]() = 30 · 0.0045 / 2 · 0.001 = 67.5 kg / mm 2 ,
= 30 · 0.0045 / 2 · 0.001 = 67.5 kg / mm 2 ,
![]() R = 30/2 = -15 kg / mm 2 , and
R = 30/2 = -15 kg / mm 2 , and
![]()
![]() -
-
![]() R = 82.5 kg / mm 2 .
R = 82.5 kg / mm 2 .
The voids between microspheres do not exceed 20% of the volume, therefore, the volume of microspheres remains 80% . The volume of the microsphere is 4/3
![]() R 3 . The volume of the internal cavity of the microsphere with hydrogen is equal to = 4/3
R 3 . The volume of the internal cavity of the microsphere with hydrogen is equal to = 4/3 ![]() 64 microns . The volume of the microsphere shell is 4/3
64 microns . The volume of the microsphere shell is 4/3
![]() (125-64) = 4/3
(125-64) = 4/3
![]() 61 . Thus, the volume of the microsphere shell and the volume with hydrogen are practically the same and each is 40% . The amount of hydrogen at 3000 atm in the microspheres in 1 liter of such granules is 1 l · 0.4 · 3000 = 1200 l . This is 3 times higher than in 1 liter capacity in the prototype at 400 atm.
61 . Thus, the volume of the microsphere shell and the volume with hydrogen are practically the same and each is 40% . The amount of hydrogen at 3000 atm in the microspheres in 1 liter of such granules is 1 l · 0.4 · 3000 = 1200 l . This is 3 times higher than in 1 liter capacity in the prototype at 400 atm.
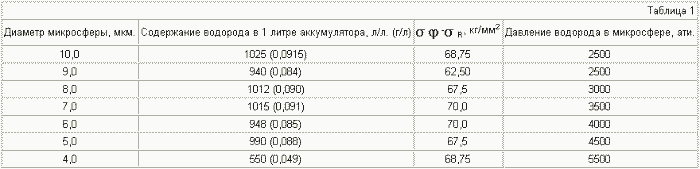
Depending on the size of the microsphere, it is possible to vary the ratio of the volume occupied by the shell and the volume occupied by hydrogen, but at the same time the pressure of hydrogen that the microsphere can withstand varies. Thus, calculations show that with increasing diameter, the proportion of the volume of hydrogen increases, but the pressure that the microsphere can withstand decreases. Table 1 shows a calculation showing the dependence of the hydrogen content in microspheres as a function of their diameter for steel EP-222 (
![]() 0.2 = 37 kg / mm 2 ,
0.2 = 37 kg / mm 2 ,
![]() Bp = 70 kg / mm 2 ) .
Bp = 70 kg / mm 2 ) .
For steels with ![]() -
-
![]() R = 150 kg / mm 2 , the hydrogen content increases almost proportionally.
R = 150 kg / mm 2 , the hydrogen content increases almost proportionally.
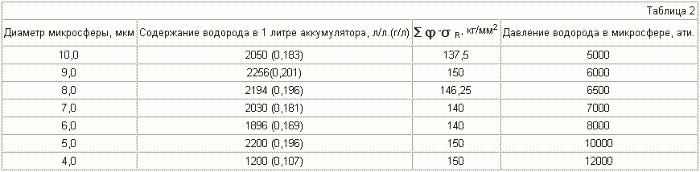
Tables 3-6 for microspheres of different diameters show data on the hydrogen content depending on the pressure inside the microspheres.
The diameter of the microspheres is 5 μm , the shell thickness is 1 μm .
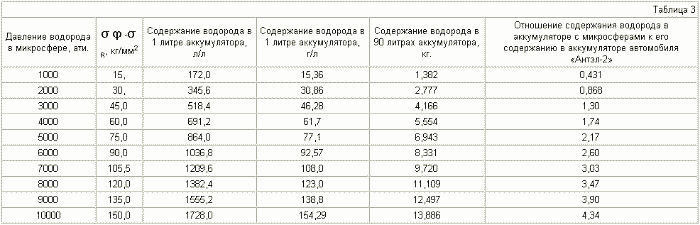
The diameter of the microspheres is 8 mkm , the thickness of the shell is 1 mkm .
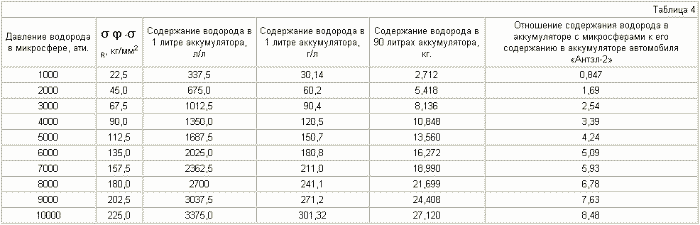
The diameter of the microspheres is 10 μm , the thickness of the shell is 1 μm .
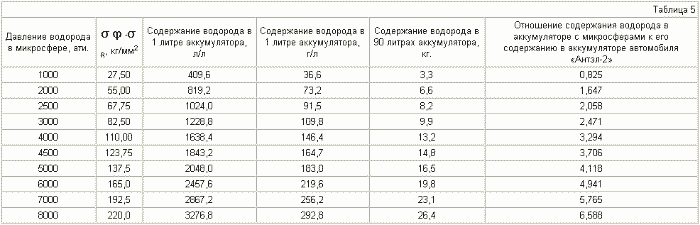
The diameter of the microspheres is 15 mkm , the thickness of the shell is 1 mkm .
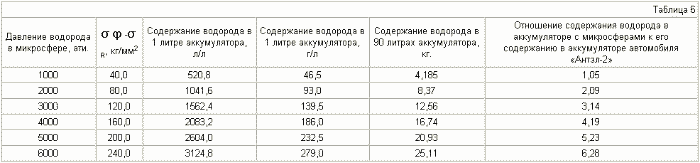
It can be seen from Table 3-6 that if the various microspheres are saturated with hydrogen to the same pressure, for example up to 2000 atm, the stresses arising in the shell of the microspheres will be different. For microspheres with a diameter of 5 μm, they are equal to 30 kg / mm 2 , for 8 μm - 45 kg / mm 2 , for 10 μm - 55 kg / mm 2 , for 15 μm - 80 kg / mm 2 . Thus, placing (pressurizing, welding) the microspheres in a container in such a way that microspheres with a large diameter are in the center of the container and they decrease to the periphery, we obtain a battery in which the magnitude of the stresses decreases as when removing from the center of the accumulator due to a reduction in the radius of the microspheres , And decreases in each microsphere due to contact of their walls with each other (we obtain a wall of double thickness with practically equal hydrogen pressure on both sides), which will reduce the probability of rupture of microspheres and the container itself.
Table 7 presents the mass contents of hydrogen depending on the hydrogen pressure in the microspheres for three materials: steel - d = 8 g / cm 3 , titanium - d = 4.5 g / cm 3 , graphite d = 2.25 g / cm , Where d is the specific weight of the material, g / cm 3 .
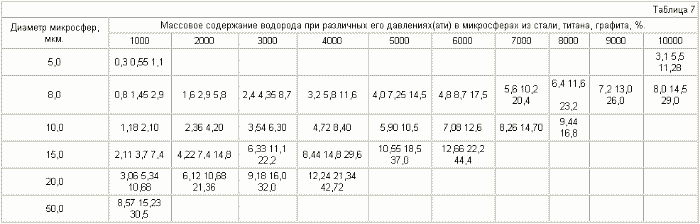
It can be seen from tables 5.7 that it is realistic to provide in the accumulator a mass content of hydrogen of 6%, at which automobile firms are ready to switch to hydrogen fuel, for microspheres with a diameter of 15 μm , using steel with ![]() Time
Time
![]() 120 kg / mm 2 , or titanium with
120 kg / mm 2 , or titanium with
![]() Time
Time
![]() 80 kg / mm, or graphite with
80 kg / mm, or graphite with
![]() Time
Time
![]() 40 kg / mm 2 .
40 kg / mm 2 .
All the above calculations and experiments show that for microspheres with a diameter in the range of 1-50 μm and a shell thickness of about 1 μm, for a wide class of high-strength metals, alloys, composite materials, it is really possible to provide a mass content of hydrogen, which becomes economically advantageous.
Example 1
Microspheres 10 μm in diameter from EI-647 steel - in the amount of 2 ml , being a negative electrode, were saturated with hydrogen at room temperature in a 4% aqueous solution of sulfuric acid. The electrolysis process lasted for 1 hour with a potential at the electrodes exceeding the water decomposition potential (more than 2V ). After the end of the process, the granules were washed with desalted water, dried in a stream of air at room temperature.
To determine the amount of accumulated hydrogen, the pellets were loaded into a sealed ampoule with a manometer. The granules were heated to 300 ° C , then the granules cooled to room temperature, the hydrogen pressure in the ampoule was measured. Measurements and calculations showed that 2,400 ml of hydrogen was released from 1 ml of microspheres, this value is close to the calculated value and corresponds to the hydrogen pressure in granules of 6,000 ati , see Table 5. , Which corresponds to a mass content of 7.1% .
Example 2
A similar experiment was carried out with microspheres 10 μm in diameter , made of a titanium alloy AT-3 . From 1 ml of microspheres, 1200 ml of hydrogen were isolated, which corresponds to a mass content of 6.3% .
Example 3
In order to intensify the process of saturation of microspheres with hydrogen, a coating of palladium with a thickness of the order of 0.1 μm was applied chemically to the surface of the microsphere by chemical means. The process of hydrogen saturation accelerated 3-4 times to its analogous content in microspheres without coating.
Example 4
In an ampoule with methane in which 10-20 kV voltage was applied to the electrodes and a quiet discharge was created to ionize the molecules, microspheres of EI-647 steel with a diameter of 15 μm were placed. Microspheres were a negative electrode, the positive electrode was made of graphite (an independent electrical circuit). Hydrogen ions from the discharge came to the negative electrode-microspheres and saturate them with hydrogen. The saturation time was 30 minutes. Then, as in Example 1, the microspheres were loaded into a sealed ampoule with a manometer and hydrogen was extracted from the microspheres. In 1 ml of microspheres contained 2250 ml of hydrogen, which corresponds to the hydrogen pressure in microspheres of 4500 ati , which is equivalent to the mass content of hydrogen in microspheres of the order of 9.2% .
The obtained results show that for these materials the calculated and experimental data are close in their values. The process of saturation of microspheres with hydrogen is realized at low temperatures. Thus, the present invention will provide the industry with a safe and economically advantageous method and capacity for storing and storing hydrogen that can be used for installation on vehicles, and used in other industries.
CLAIM
1. A hydrogen storage container consisting of a sealed enclosure, process connections, an internal heat exchange surface and a hydrogen storage accumulator disposed in the housing, characterized in that the hydrogen filler-accumulator is hollow microspheres of a conductive material with a tensile strength of more than 30 kg / Mm 2 .
2. A container according to claim 1, characterized in that the material of the microspheres is steel or titanium, or lanthanum, or nickel, or zirconium, or alloys based on these metals, or graphite, or graphite-based compositions.
3. A container according to claim 1, characterized in that the diameter of the microspheres decreases from the center of the body to the periphery.
4. A container according to claim 1, characterized in that the microsphere has a coating of a metal absorbing hydrogen, for example palladium or nickel, or an alloy of lanthanum with nickel.
5. A container according to claim 1, characterized in that the body is made of a non-conductive material with a negative electrode disposed inside and has a branch pipe for supplying a hydrogen-containing medium, the positive electrode being located outside the housing.
6. A method for hydrogen storage consisting of saturation of microspheres by hydrogen diffusion, characterized in that microspheres being a negative electrode are placed in a hydrogen-containing medium, and the saturation of the microspheres is carried out with hydrogen converted into an ionic form.
7. The method of claim 6, wherein the transfer of hydrogen to the ionic form is carried out by electrolysis in aqueous solutions.
8. The method of claim 6, wherein the transfer of hydrogen to the ionic form is carried out by ionization, for example, in an electric discharge.
print version
Date of publication 11/28/2006




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.