| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2228485
![]()
The method and apparatus for storing GAS SUBSTANCE, absorbing gas,
AND METHOD FOR THE PRODUCTION THEREOF
Name of the inventor: Okazaki Toshihiro (JP); Naoki Nakamura (JP); Takuya Kondo (JP); Masahiko Sugiyama (JP)
The name of the patentee: TOYOTA DZIDOSYA Kabushiki Kaisha (JP)
Patent Attorney: Vysotskaya Nina
Address for correspondence: 103735, Moscow, ul. Ilinka, 5/2 "Sojuzpatent" pat.pov. N.N.Vysotskoy
Starting date of the patent: 1999.06.30
Gas accumulation method includes three stages. The first stage - accumulated by the exposure to the gas container and the adsorbent at a low temperature below the liquefaction temperature of the gas accumulated by. Thus accumulated gas is adsorbed in the adsorbent in a liquefied state. The second step - the introduction into the vessel for storage at a low temperature a gaseous or liquid medium with a freezing temperature which is higher than the above-mentioned liquefaction temperature of the gas accumulated by the freezing of the medium. When the gas that has been adsorbed in the adsorbent in a liquefied state is encapsulated this frozen medium. And the third stage - this aging tank at a temperature higher than the liquefaction temperature and below the freezing temperature. As the getter material used may be selected from the group consisting of antrotsensoderzhaschih planar molecules ftalotsianinsoderzhaschih cyclic molecules, the cyclic molecules paratsiklofansoderzhaschih kronefirsoderzhaschih and cyclic molecules. A getter material may further comprise a spherical molecule. Use of the invention will improve the storage density of gas during adsorption without using low temperatures and apply getter material with a high storage efficiency.
DESCRIPTION OF THE INVENTION
The invention relates to a method and apparatus for gas storage, namely natural gas, by absorbing substance and absorbing the gas on the basis of adsorption, and to a process for its preparation.
An important issue for accumulation of gas, such as natural gas is that the gas which has a low density at normal temperature and pressure can be accumulated efficiently at high density. Even natural gas components such as butane and similar gases can be liquefied at normal pressure due to compression at relatively low pressure compressed natural gas (CNG), but methane and similar gases are not easily liquefied by means of the pressure at ordinary temperature.
The first method, which is commonly used as a method for storage of such gases which are difficult to liquefy by pressure at near normal temperature, is liquefaction while maintaining the low temperature as in the case of LNG (liquefied natural gas) and the like. With this liquefaction process it is possible to accumulate a 600-fold volume at normal temperature and pressure. However, for example, LNG, the need to maintain the cryogenic temperature below -163ºS or complexity inevitably leads to increase in the cost of equipment and operating costs.
An alternative storage method is studied by gas adsorption (ANG: adsorbed natural gas) without special pressure and low temperature.
In Japanese Examined Patent Publication number 9-210295 proposed an adsorption storage method for gas such as methane and ethane in a porous material such as activated carbon at near normal temperature, in the presence of a basic compound such as water, and this publication explains that large-scale accumulation of gas by volume is possible due to the combined effect of the adsorption capacity and the effect psevdovysokogo pressure of the porous material and the formation of inclusion compounds in the above basic compound.
However, even this proposed method is not able to create a storage density comparable to that achieved by using the methods of storage of cryogenic temperature, a gas such as LNG.
It has been proposed to use activated carbon as the getter material (substance) for accumulation of gases that do not liquefy at relatively low pressures up to 10 atmospheres, such as hydrogen and natural gas (see., E.g., laying open a patent application in Japan for № 9-86912 ). Activated carbon can be manufactured based on coconut shell, wood fibers, coal, etc. and the like, but it has a reduced storage efficiency (volume of gas accumulation capacitance per unit volume) in comparison to conventional methods of accumulation of gas, such as compressed natural gas (CNG) and liquefied natural gas (LNG). This is because among the various pore sizes of the pores of the activated carbon is only a limited size effectively function as adsorption sites. For example, methane is adsorbed only in micropores (2 nm or less), while pores of other sizes (mesopores: approximately 2-50 nm, macropores: 50 nm and greater) contribute little methane adsorption.
In the above patent RU № 2148204 of 27.04.2000, the described vehicle for the transport of units for storage of liquefied gas fuel consisting of fuel gas supply station, a gas storage tank installed on a vehicle, and the adsorbent placed in the container. However, this device does not provide a very high density storage by adsorption gas and requires very low temperatures in the tank.
The main object of the present invention is to provide a method and a gas storage installation, which can provide very high density storage by adsorption, without the use of low temperatures.
A second object of the present invention to provide a getter material (substance) with higher storage efficiency than activated carbon.
In accordance with a first aspect of the present invention for achieving the aforementioned first purpose envisaged to develop a method of gas storage, comprising:
space accumulated by the gas adsorbent into the container at a low temperature below the liquefaction of the respective accumulated by the gas temperature, so that said gas intended to be stored, adsorbed into said adsorbent in a liquefied state,
introducing into said vessel for storage at a low temperature a gaseous or liquid medium at a temperature of cooling, which is higher than the above-mentioned liquefaction temperature of said accumulated by gas for freezing of said medium, so that the accumulated gas that was adsorbed in the adsorbent in a liquefied condition, included in this medium, which was frozen, and
placing this vessel at a temperature higher than the liquefaction temperature and corresponding appropriate lower freezing temperature.
According to a first aspect of the invention provides, in addition, the installation of gas storage, characterized by including:
- gas supply source which supplies gaseous or liquefied gas,
- gas storage capacity,
- adsorbent is enclosed in a container,
- Device for placing said contents of the vessel at a low temperature below the liquefaction of the corresponding temperature,
- a gaseous or liquid medium with a freezing temperature which is higher than the liquefaction temperature of the respective gas,
- Device for placing said contents of the vessel at a temperature higher than the liquefaction temperature of the corresponding, but lower than the corresponding freezing temperatures,
- means for introducing this gas from said gas supply source in a vessel and means for introducing the medium in a tank.
According to a first aspect of the invention provides, moreover, to create a vehicle for the transport of liquid accumulated by the installation of the fuel gas, which includes:
- Liquid filling station fuel gas,
- container for storing fuel gas, placed in a vehicle for transportation,
- absorbent, enclosed in a container,
- said device for holding the contents of the vessel at a low temperature below the liquefaction of the corresponding temperature,
- gaseous or liquid medium with a freezing temperature which is higher than the liquefaction temperature of the respective fuel gas,
- a temperature device for holding the contents of the said tank is higher than the liquefaction temperature and corresponding lower than said freezing temperature,
- means for introducing gas of fuel from said fuel gas filling stations in said vessel and means for introducing said medium in a container.
According to a second object of the present invention for achieving the above second object is provided to create a gas occluding material comprising any of the two planar molecules and cyclic molecules. It may include globular molecules.
At this getter material of the present invention the gas is adsorbed between the planes of said planar molecules or in the nucleus of said cyclic molecules. Advantageously, the cyclic molecular core size was slightly larger than the size of the gas molecules.
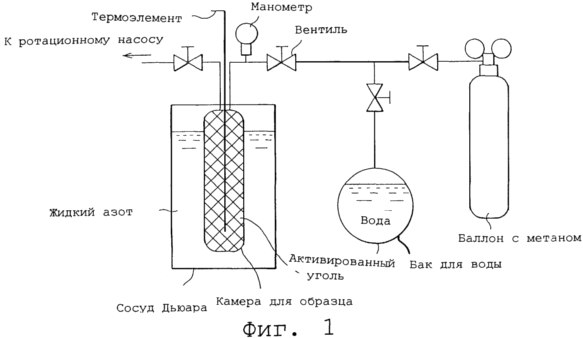
1 shows a diagram representing, in accordance with the present invention, a construction example of an apparatus for gas storage.
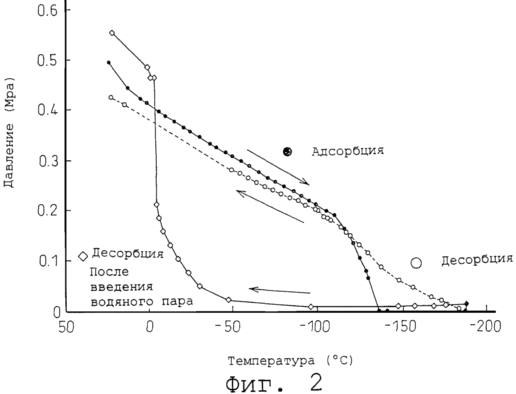
2 is a graph showing a comparison example of the present invention and the comparative example, values expressed in a temperature-dependent nature of the desorption of methane gas adsorbed and liquefied at a cryogenic temperature.
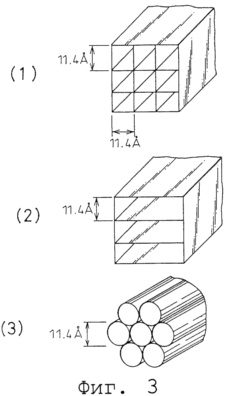 |
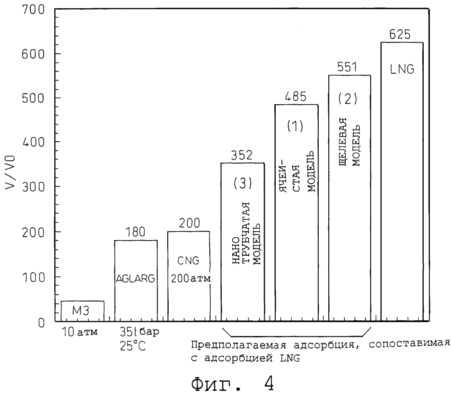 |
Figure 3 (1) -3 (2), in accordance with the present invention, are schematic drawings depicting examples theoretical structural model getter substances.
4 is a graph showing comparison of storage efficiency V / V0 volume for different structural models shown in Figure 3, and conventional gas storage systems.
 |
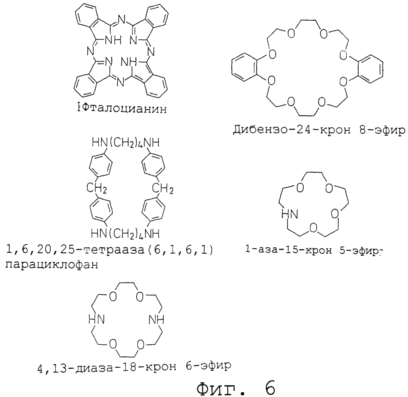 |
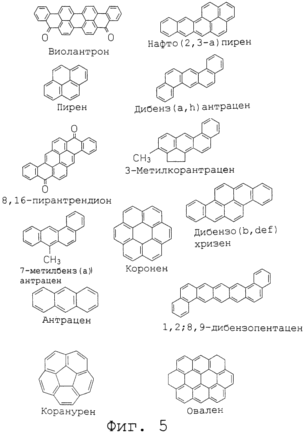
Figure 5 shows the structural formulas of the typical planar molecules.
Figure 6 shows the structural formulas of typical cyclic molecules.
 |
 |
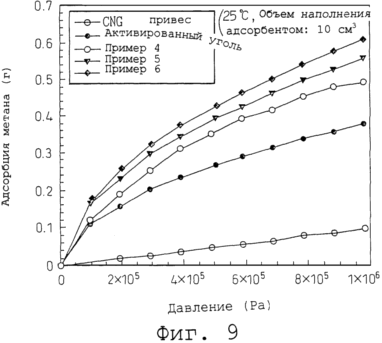 |
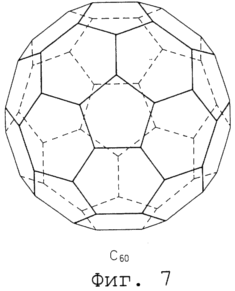
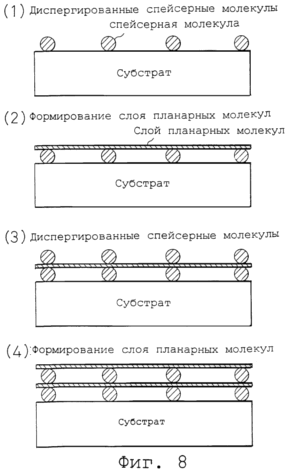
Figure 7 shows the structural formula of a typical spherical molecule. 8 is a set of conceptual drawings of the variant formation of planar molecule layer and dispersion of globular molecules. 9 is a graph showing the results measuring methane adsorption under various pressures, for a gas occluding material according to the present invention and a conventional getter material. In accordance with the main aim of the present invention a gas which is in a liquid state at low temperature, it is encapsulated using a frozen environment, which provides for the accumulation of freezing at a temperature higher than this necessary cryogenic temperature for liquefaction. The gas injected into storage for the storage container in the gaseous or liquid state. In gas for storage, that is injected in a gaseous state must first be lowered to a cryogenic temperature for liquefaction, and then to encapsulate in a liquefied state in a frozen environment where it can be stored frozen at a temperature higher than this cryogenic temperature. |
Used frozen medium is a substance which is gaseous or liquid, and has a higher freezing temperature than the liquefaction temperature of the corresponding gas for storage, and does not interact with the gas intended for the storage, according to an adsorbent or a given capacity at a given temperature storage .
Through the use of a medium with a freezing temperature (melting temperature, sublimation temperature) close to room temperature, it is possible to carry out accumulation at near room temperature, maintaining, however, the high density exhibited by at cryogenic temperature.
Representative examples of such media are substances with a freezing temperature (commonly, "melting temperature") in the range from -20 to + 20ºC, such as the water (Tm = ºC), dodecane (-9,6ºC), dimethyl phthalate (0ºC) , diethyl phthalate (-3ºC), cyclohexane (6,5ºC) and dimethyl carbonate (0,5ºC).
Traditionally used adsorbent can be a gas adsorbent, typical of which are various inorganic or organic adsorbents such as activated carbon, zeolite, silica gel, etc.
For gas storage can be any gas that can be liquefied and adsorbed at a cryogenic temperature comparable to conventional LNG or liquid nitrogen, or hydrogen, helium, nitrogen can be used, and hydrocarbon gases. Typical examples of hydrocarbon gases include methane, ethane, propane, etc.
Structural examples of theoretical models of getter materials according to the second aspect of the present invention are shown in Figure 3. Based on the diameter of the carbon atoms of 0.77 ![]() and distance C-C bond in the 1,54
and distance C-C bond in the 1,54 ![]() it is possible to construct gaps of ideal size for adsorption of molecules of the target gas. In the illustrated example, the size of the gaps 11.4
it is possible to construct gaps of ideal size for adsorption of molecules of the target gas. In the illustrated example, the size of the gaps 11.4 ![]() It was used as an ideal for the adsorption of methane.
It was used as an ideal for the adsorption of methane.
Figure 3 (1) shows a honeycomb structure model, having a square cross-grid form with sides 11.4 ![]() and pore volume of 77.6%.
and pore volume of 77.6%.
Figure 3 (2) shows a structural model of a slit having a slit width layered 11.4 ![]() and pore volume of 88.1%.
and pore volume of 88.1%.
Figure 3 (3) shows the nanotube structural model (for example, 53 carbon tubes, single wall) with the construction of a bundle of carbon nanotubes with a diameter of 11.4 ![]() and pore volume of 56.3%.
and pore volume of 56.3%.
4 shows the effective storage volume V / V0 getter substances for different structural models shown in Figure 3, compared with the conventional storage methods.
Typical planar molecules used to construct the absorbent material (substance), in accordance with the present invention include coronene, anthracene, pyrene, naphtho (2,3-a) pyrene, 3-metilkonantren, violantron 7-methylbenz (a) anthracene, dibenz (a, h) anthracene, 3-metilkorantratsen, dibeno (b, def) chrysene, 1,2; 8,9-dibenzopentatsen, 8.16-piranantrendion, koranuren and ovals. Their structural formulas are shown in Figure 5.
Conventional cyclic molecules include phthalocyanine, 1-aza-15-crown 5-ether, 4,13-diaza-18-crown 6-ether, dibenzo-24-crown 8-ether and 1,6,20,25-tetraaza (6 , 1,6,1,) paracyclophane. Their structural formulas are shown in Figure 6.
Typical globular molecules used are fullareny which include C 60, C 70, C 76, C 84, etc. etc. as the number of carbon atoms in the molecule. As a typical example in Figure 7 shows the structural formula of C 60.
When globular molecules are included, in particular between planar molecules, they function as spacers to form a gasket 2,0-20 ![]() That have a suitable size for adsorption of gas molecules such as hydrogen, methane, propane, CO 2, ethane, etc. For example, have a diameter in fullareny 10-18
That have a suitable size for adsorption of gas molecules such as hydrogen, methane, propane, CO 2, ethane, etc. For example, have a diameter in fullareny 10-18 ![]() and are particularly successful for the formation of microporous structures appropriate for adsorption of methane. Spherical molecule is added in an amount of about 1-50% to achieve a spacer effect.
and are particularly successful for the formation of microporous structures appropriate for adsorption of methane. Spherical molecule is added in an amount of about 1-50% to achieve a spacer effect.
A preferred form of getter material according to the present invention is a powder form, and a suitable vessel may be filled with powder material from the spherical molecules, powder material from cyclic molecules, a mixture of both powders, or any one of three kinds of powder mixed with powder material of spherical molecules.
Processing capacity of ultrasonic vibrations is preferred to increase the filling density, which, however, increase the degree of dispersion, to help prevent aggregation of these molecules.
Another preferred form of the getter material according to the present invention is to form alternating layers of planar molecules and globular molecules. This embodiment is preferred for said spherical molecules are sprayed with spraying. Such intermittent formation of layers of planar molecule / globular molecules by means of conventional lamination techniques formation, such as vacuum sputtering electron beam, molecular beam epitaxy (MBE) or laser ablation.
8 schematically illustrates a sequential process for alternate layer-formation. Initially, in step (1) said spacer molecules (globular molecules) are sprayed onto the substrate. This can be accomplished, for example, by placing, achieved by spraying in a dispersion medium data spacer molecules (a volatile solvent such as ethanol, acetone, etc.). The layer of spacer molecules can be formed by a vacuum layer formation method such as MBE, laser ablation or a similar, using fast evaporation under vacuum layer formation rate (1 ![]() / Sec or less) that is lower than the rate of formation of the monomolecular layer. Then, in step (2), the planar molecules are accumulated by the method of forming the respective layers so that the individual planar molecules bridge across lined plurality of spherical molecules. This forms a planar molecule layer manner which maintains an open space from the surface of the substrate. In step (3), the spacer molecules are distributed in the same manner as in step (1) on the planar molecule layer formed in step (2). Then in step (4), a planar molecule layer formed in the same manner as in step (2). These steps are then repeated to form a getter material (substance) of the desired thickness.
/ Sec or less) that is lower than the rate of formation of the monomolecular layer. Then, in step (2), the planar molecules are accumulated by the method of forming the respective layers so that the individual planar molecules bridge across lined plurality of spherical molecules. This forms a planar molecule layer manner which maintains an open space from the surface of the substrate. In step (3), the spacer molecules are distributed in the same manner as in step (1) on the planar molecule layer formed in step (2). Then in step (4), a planar molecule layer formed in the same manner as in step (2). These steps are then repeated to form a getter material (substance) of the desired thickness.
Applicable molecular-planar layer may consist of any of the above molecules or planar layered materials such as graphite, boron nitride, etc. And can be used with a multilayer structure materials such as metals and ceramics.
example 1
In accordance with the present invention apparatus, the structure of which is shown in Figure 1 was used for storage of methane gas according to the following procedure.
Initially, 5 g of activated carbon powder (particle size approximately 3-5 mm) was loaded into a sample capsule (volume 10 cm3) having a sealed construction, and the pressure inside the capsule was reduced by a rotary pump to 1 x 10 -6 MPa .
Then, the capsule was administered methane from methane with cylinder actuation internal capsule pressure to 0.5 MPa.
This capsule in this state was immersed in liquid nitrogen in a Dewar vessel, and kept there for 20 minutes at the temperature of liquid nitrogen (-196ºC). This entire operation is liquefied methane gas in the capsule and adsorbed it in the activated carbon.
This capsule is continuously kept immersed in the liquid nitrogen, and water vapor formed in the tank with water (20-60ºC temperature) was injected into the desired capsule. This caused immediate freezing of the water vapor to ice by the temperature of liquid nitrogen so that the liquefied and adsorbed methane gas was frozen and encapsulated in the formed ice.
In Comparative Example stage liquefaction and adsorption of methane gas was carried out according to the same procedure as in Example 1 but the steam is not injected.
Figure 2 shows the character of methane desorption at these temperatures capsules accumulating methane, according to Example 1 and Comparative Example naturally temperature allowed to rise to room temperature. In the drawing on the horizontal axis shows temperatures and the vertical axis, respectively, delayed pressure readings indicated temperature and pressure in the capsule was measured using thermocouple and pressure gauge shown in Figure 1.
<Process of adsorption and liquefaction: For Example 1 and Comparative Example (Figure 2 ·)>
If the capsule-introduced methane was immersed in liquid nitrogen, adsorption proceeds as the temperature drop inside the capsule, falling due to the line pressure drop therein, and when liquefaction begins intracapsular pressure falls quickly to 0 MPa, despite the fact that the temperature liquid nitrogen reaches -196ºC.
<Desorption process: Comparison between Example 1 and Comparative Example>
In Comparative Example (0 to 2), wherein the steam is not introduced after the liquid nitrogen temperature, the resulting temperature increase resulting likelihood of creating conditions in which already a slight increase in the temperature starts to run -180ºC methane desorption process, and initiates the pressure increase.
In contrast, in this example, (by ![]() 2) difference is that, in accordance with the present invention, water vapor is introduced on reaching a temperature of liquid nitrogen, to perform the freezing encapsulation, the desorption detected the presence of increasing the volume of the compression exercised only after raising the temperature to -50 º C, and the basic portion of the methane is adsorbed and desorbed even at 0ºC.
2) difference is that, in accordance with the present invention, water vapor is introduced on reaching a temperature of liquid nitrogen, to perform the freezing encapsulation, the desorption detected the presence of increasing the volume of the compression exercised only after raising the temperature to -50 º C, and the basic portion of the methane is adsorbed and desorbed even at 0ºC.
example 2
gas accumulation performed in accordance with the present invention by the same procedure as in Example 1, except that the capsule, upon reaching the temperature of liquid nitrogen, instead of steam from the water tank the water injected in the liquid state.
The result showed the same character desorption as in Example 1, as shown in Figure 2, a low pressure is maintained almost at 0ºC.
example 3
The apparatus structure shown in Figure 1 was used for storage of methane gas, to carry out the invention according to the following procedure. However, the accumulated gas was a liquefied methane supplied from a liquefied methane vessel, instead of with the methane gas from the cylinder with methane.
Initially, 5 g of activated carbon powder (particle size approximately 3-5 mm) was loaded into a sample capsule (volume 10 cm3) having a sealed structure.
This capsule was immersed directly into a Dewar vessel filled with liquid nitrogen, and kept at the temperature of liquid nitrogen (-196ºC) for 20 minutes.
Then injected into the capsule from the liquefied methane vessel with liquid methane. This resulted in adsorption of the liquefied methane charcoal loaded into the capsule.
Then, the capsule was immersed in a liquid nitrogen environment, and the steam produced in the water tank (20-60ºC temperature), admitted into the capsule. This caused immediate freezing of the steam let in to ice by the temperature of liquid nitrogen, so that the liquefied and adsorbed methane gas was frozen and encapsulated in the formed ice.
example 4
In accordance with the present invention, the getter material was prepared with the following composition:
The cyclic molecule: 1,6,20,25-tetrazene (6,1,6,1) paratsiklofanovy powder.
example 5
In accordance with the present invention, the getter material was prepared with the following composition:
Planar molecule: 3-metilkorantratsenovy powder content of 90 wt.%.
Spherical molecule: C 60 -vy powder content of 10 wt.%.
example 6
A gas occluding material according to the present invention, prepared in Example 5 was placed in a container and treated by ultrasonic vibrations at a frequency of 50 Hz for 10 minutes.
Methane adsorption getter materials prepared in Examples 4-6 above were measured under various pressures. For example, the same measurement was performed for activated carbon (mean particle size: 5 mm) and CNG. The measurement conditions were as follows:
25ºC temperature;
volume, filled with an adsorbent 10 cm 3.
The result found that, as shown in Figure 9, getter materials prepared in Examples 4-6, according to the present invention performed better methane adsorption than activated carbon. Furthermore, in Example 5, which was supplemented with spherical molecules, and in Example 6, which used an ultrasonic treatment was observed even better adsorption than Example 4. That is, in Example 5 maintained suitable gaps due to the effect of spherical spacer molecules that manifest a higher adsorption than Example 4. Example 6 and observed a better filling density and the degree of dispersion associated with the use of ultrasonic waves and showing, for this reason, even higher adsorption than Example 5.
In accordance with the main object of the present invention we have developed a method and a gas storage device that can realize a very high density storage by adsorption, without the use of low temperatures.
Since the method of the present invention does not require low temperatures during accumulation, it can be satisfactorily carried out in a conventional freezer, operating from -10 to about 20ºC, and thus equipment and operating costs can be reduced.
Moreover, a tank for storage, and other equipment do not need a special construction in materials required for low temperature, and hence provide an advantage in terms of the costs for material.
According to a second object of the present invention, a gas occluding material with higher storage efficiency than activated carbon.
CLAIM
1. A method for gas storage, consisting in that gas is accumulated and kept in the adsorbent vessel at a low temperature below the liquefaction temperature of said gas, which accumulates and is fed into a tank filled with an adsorbent in a gaseous or liquefied state, wherein the accumulated gas adsorbed in the adsorbent in a liquefied state, is introduced into a container for holding at a low temperature a gaseous or liquid medium at a freezing temperature which is higher than the temperature for liquefying accumulated by gas for freezing of said medium, so that the accumulated gas is adsorbed in the adsorbent in a liquefied state , was included into the medium which has been frozen, and then said vessel maintained at a temperature higher than the liquefaction temperature and below the freezing temperature.
2. Installation for the storage of gas, which consists of a gas supply source which supplies gaseous or liquefied gas, a gas storage tank filled with an adsorbent device for holding the contents of said vessel at a low temperature below the liquefaction temperature of the gas, characterized in that it comprises a gaseous or liquid medium with a freezing temperature which is higher than the liquefaction temperature of said gas, a device for holding at a temperature of the contents of the container higher than the liquefaction temperature, but lower than the freezing temperature, means for introducing gas from the source of its supply to the vessel and the device for introducing said medium into the vessel.
3. A vehicle for the transport of units for storage of liquefied gas fuel consisting of fuel gas supply station, a gas storage tank installed on a vehicle filled with an adsorbent, characterized in that it comprises a device for holding the contents of the container at a low temperature below the liquefaction temperature said gas, a gaseous or liquid medium with a freezing temperature which is higher than the liquefaction temperature of said fuel gas, a device for holding the contents of said vessel at a temperature higher than said liquefaction temperature, but lower than said freezing temperature, a device for introducing said fuel gas from the fuel gas supply station into the vessel and means for introducing said medium into said vessel.
4. A getter material consisting of anthracene molecules, characterized in that further includes globular molecules.
5. A getter material consisting of cyclic molecules, wherein the cyclic molecule is selected from the group consisting of cyclic ftalotsianinsoderzhaschih molecules paratsiklofansoderzhaschih kronefirsoderzhaschih cyclic molecules and cyclic molecules, and further includes globular molecules.
6. A getter material of claim 5, characterized in that the molecules are spherical fullerenes.
7. A method of producing a gas occluding material based powders, characterized in that the ultrasonic wave is treated vessel filled with powder from the planar-molecular substance, a powder of a cyclically-molecular powder mixture of both powders contained in the container.
8. A method according to claim 7, characterized in that the powder of planar molecular substance powder cyclically-molecular substance or a mixture is further mixed in the container with a powder of spherically-molecular substance to increase the filling density and the degree of dispersion.
9. A method of producing a gas occluding material comprising porous material laminated formation with alternating layers of getter materials with different pore sizes and with different degrees of dispersion, characterized in that the layers consist of planar molecules and globular molecules, wherein the globular molecules are dispersed by spraying.
10. A gas storage method as claimed in claim 1, characterized in that used as the adsorbent getter material according to any one pp.4-9.
11. The gas storage installation according to claim 2, characterized in that used as the adsorbent getter material according to any one pp.4-9.
12. A vehicle according to claim 3, characterized in that used as the adsorbent getter material according to any one pp.4-9.
print version
Publication date 22.12.2006gg





Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.