| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2070186
![]()
METHOD fertilized soil and fertilizer for the soil
Name of the inventor: William O.Stauffer [US]; F.Robert Hubbard [US]
The name of the patentee: IMCO Recycling Inc.. (US)
Address for correspondence:
Starting date of the patent: 1992.08.12
The invention relates to a process for producing fertilizer from aluminum scrap, but also to methods for fertilizing soil using spent flux from aluminum recovery process. fertilizers method comprises introducing into it a mineral nutrient mixture consisting of potassium salts and trace elements. The nutrient mixtures used aluminum-containing waste pretreated with molten flux and subsequent separation of the aluminum salt phase while the salt phase is crushed to a particle size not exceeding 10 mm. Aluminum waste contains aluminum oxide, salts, oxides or nitrides of barium, calcium, copper, iron, magnesium, manganese and titanium, and aluminum nitride, and the molten flux contains 90-95 wt.% Of potassium chloride. Fertilizer for the soil is a mixture of potassium-based. The fertilizer is obtained by treating aluminum-containing material with molten flux. In the flux may be added other salts and other materials.
DESCRIPTION OF THE INVENTION
The invention relates to a method for fertilizing soil and to obtain fertilizer for soil.
With a constant and even growing interest in the recovery of materials from household and industrial waste industry of secondary aluminum production industry continues to be viewed as an important and significant power saving and profit maximization of valuable natural resources. In the industry, producing secondary aluminum, various methods and technologies to recover usable aluminum metal from various types of aluminum scrap and other waste containing aluminum.
One such method is the melting of the aluminum contained in the waste, in which the flux material is contacted with a molten salt. As the salt used 100% NaCl or NaCl and up to 60% KCl. The preference for NaCl, can be attributed to its low cost, and the inclusion of KCl is a means of reducing the melting point of the flux. In either case, with sufficient agitation and a sufficiently fluid flux, the molten salt wets the impurities in the waste material and thereby causes the aluminum to separate from both the flux and the impurities originally present in the aluminum. The flux protects the molten aluminum to protect it against oxidation in the furnace itself. The separated aluminum forms droplets which are amalgamated to form a liquid aluminum bath beneath the molten salt, and this pool is readily removed as high purity aluminum for use in rolling mills for the production of aluminum sheet metal alloys and any other types of aluminum processing.
The remaining flux together with residual impurities is usually placed in the dumps that match on environmental standards. But still remains the possibility of environmental pollution, since NaCl may contaminate aquifers and groundwater. Furthermore, the number of dumps to the respective seats is reduced and the cost of using these sites continues to increase.
A method of producing a granular fertilizer magnesium salt waste from manufacture containing potassium chloride, magnesium, sodium, calcium. The disadvantage of this fertilizer is poor digestibility elements crops and therefore does not contribute to the latter yields (1).
Known and the method of increasing the yield of crops by soil application of fertilizers based on potassium salts and trace elements necessary for plants (2).
However, in sandy, loamy and podzolic soils, these fertilizers are ineffective, since the chloride ions contained in the fertilizer, have a detrimental effect on the culture and cause a decrease in the yield of these crops.
The object of the invention to provide the possibility of using production wastes as potassium fertilizer without damaging the crops and thus increasing the yield of crops, a process for producing fertilizer from waste fluxes that would be safe and would be a useful from the point of view of environmental protection.
In accordance with the invention NaCl, normally used in the flux is replaced with KCl, the KCl serving as the major component of the flux. In specific versions of the invention KCl used to eliminate NaCl, although it may include other salts which are insoluble in water or acceptable from the standpoint of environmental protection. In any case, after the use of flux to extract impurities from the aluminum, the spent flux (or "dross") is useful as a soil fertilizer, with the potassium content of the flux serving as a source of potassium nutrient for the soil. In preferred embodiments of its use as a fertilizer spent flux is comminuted to an appropriate particle size, and, when required, it is connected together with conventional carriers, activates additives, solvents or other additives commonly added to fertilizer.
The potassium in the spent flux has a high solubility in water at any pH, while the aluminum is not. As it is generally considered that the solubility of aluminum increases with decreasing pH, it was surprising to find that the aluminum in the form in which it exists in the spent flux has essentially zero solubility at pH values above 6.0, and remains, even with limited solubility at extremely acidic conditions, well below any levels which give rise to phytotoxic effects in the soil. Thus, despite aluminum's known harmful effect on certain plants and its presence in the spent flux, the spent flux is useful as a plant fertilizer with the maximum benefit from the potassium and minimal if any effect of aluminum.
On the other hand, the advantage lies in the fact that salts which are normally unsuitable for use in the fertilizer to the soil, can now be present due to their inclusion in the flux without causing the harm they would otherwise cause. These salts are water-soluble metallic chlorides which, upon contact with soil moisture, form HCl, which is harmful to plant life. However, according to the invention, these salts are accompanied by nitrogen which is contained in the spent flux as nitrides. Upon contact of these compounds in N 2 is converted into a form in which it can be helpful as a nitrogen fertilizer, and to a level that is sufficient to completely neutralize the HCl, i.e. sufficient to exclude the possibility of harm.
Thus, the invention relates to a new fertilizer, which is a potassium-containing mixture, but also to methods of producing fertilizer from aluminum scrap, methods for fertilizing soil using spent flux from aluminum recovery process.
fertilizers method comprises introducing into it a mineral nutrient mixture consisting of potassium salts and trace elements, namely aluminum-containing waste pretreated with molten flux and subsequent separation of the aluminum salt phase while the salt phase is crushed to a particle size not exceeding 10 mm. Aluminum waste contains aluminum oxide, salts, oxides or nitrides of barium, calcium, copper, iron, magnesium, manganese and titanium, and aluminum nitride.
In accordance with the invention, KCl serves as a substitute for NaCl and may entirely replace NaCl in the flux. However, the invention and relates to fluxes which contain a minor amount of NaCl, provided that there is sufficient KCl, effectively acting as a plant nutrient when serial input spent flux into the soil, but also that the level of NaCl is low enough to avoid applying any significant damage soil. In general, for salt mixtures where both are present KCl, NaCl and preferred mixtures are those in which the KCl contained in an amount of at least about 90 wt. the total content of NaCl and KCl, and most preferably at least about 95 wt.
Other salts and other materials may be included in the flux, and in accordance with their inclusion in conventional fluxes. These materials are preferably insoluble or materials that are introduced into the soil is not objectionable from the standpoint of environmental protection. Examples of such materials is Cryolite and other natural minerals.
The aluminum materials treated by the flux prior to use as a fertilizer, in accordance with the invention may include a wide range of aluminum scrap or waste from various sources, including both industrial and domestic waste. Examples of industrial waste are sheet mill scrap such as dust from the surface layer during rolling or cladding operations, and melting furnace waste such as lumps, dross or slag. Prime examples of consumer waste are used beverage cans. Scrap of any kind will frequently include additional materials such as dirt, sand, dust and waste from the floor and furnace systems, and various foreign matter. To improve the recovery process before loading the scrap into the furnace it can be treated in different ways, for example by preheating or solvent treatment to remove printing inks and coatings, and size reduction or shredding to accelerate melting.
Restoring method in which the first flux is used, it is usually carried out in a vessel which provides for heating and mixing the materials in the molten state and which allows the molten aluminum to precipitate as a separate phase underneath the flux and removed without mixing the phases. Equipment for implementing the process and operating conditions for use with a conventional flux NaCl or NaCl / KCl are known in the art and are suitable for use in the invention. Heating can be carried out directly inside the recovery tank or container by preheating gas or oil-fired burners, inductive heaters or resistance heaters. Mixing may be achieved by agitators or stirrers or by rotation of the vessel itself. The operation can be performed on a periodic or continuous basis.
It is possible to use any type of furnace. Examples are negative, rotary kilns, furnaces for melting out and melting furnaces with side loading. Rotary drum furnace disclosed by Evans et al., U.S. Patent N 4,337,929, issued July 6, 1982 The typical furnace has a capacity of between 3,000 and 25,000 pounds, depending on the location of its placement, gas and oil availability, and other factors, and respectively set oven and is connected to the collection systems and flue gas exhaust, which meet the environmental requirements.
It can be used and such operating conditions as are commonly used in the known methods of extraction of aluminum flux NaCl or NaCl / KCl. The operating temperature may vary depending on the materials used, their relative amounts, the form in which they are supplied, and the degree of contamination. In fact, the temperature may be lower than that normally used in known processes which utilize only NaCl, due to the low KCl melting temperature, but higher than that used in conventional processes where a mixture of NaCl and KCl due eutectic this combination effect . In most applications, best results are achieved at temperatures in the range of about 1000 o F to about 1700 o F (538 927 o C), preferably about 1600 o F (649 671 o C), and most preferably at about 1300 1500 o F (704 816 o C).
The contact time of the materials at the operating temperature may vary depending on many of the same factors. The contact time should be sufficient to achieve complete melting of those ingredients which will melt at the operating temperature. In conventional processes, the contact time will be between about 10 minutes and one hour per batch.
During aluminum reduction process viscosity reduction flux are preferably minimized or avoided since they may cause suspended particles to agglomerate. This effect is known in the industry as "drying" or "drying" and it can be easily detected. If drying takes place, the process can still be continued, although the output may decrease in efficiency or recovery. In any event, drying is readily eliminated or avoided by using an appropriate ratio of the flux to scrap ratio, charging additional salt to the flux when needed, selecting and controlling other operating conditions, but also to exclude other methods known to those skilled in the art.
The reduction method can be conducted in various ways. For example, in batch processes per load flux can be reused without removing impurities. Thus, a single flux charge may be used with several scrap charges.
In a typical operation, lined rotary kiln equipped with a burner operating on natural gas and / or propane burner is charged with aluminum scrap (such as sheet metal skim or used aluminum beverage containers) and the KCl salt flux on basis. After the spent flux process is completed is obtained in the form of black dross, which includes the potassium chloride and such components as, for example, aluminum oxide, aluminum chloride, silica, aluminum nitride, aluminum carbide and inerts, which were impurities in the aluminum scrap. This black dross is removed from the furnace and processed further for use as soil fertilizer. Aluminum, which is deposited on the furnace bottom is separately removed and transferred to aluminum sheet mills, or to be used as raw materials.
Peruse components of the black dross along with the original flux materials, and aluminum is present, which is usually in the form of alumina, aluminum chloride, and metallic aluminum which failed to coalesce in the liquid aluminum phase. As indicated above, aluminum is generally phytotoxic, but its inclusion in the resulting solids mixture according to this invention has no adverse merging the suitability of this mixture as a soil fertilizer since the aluminum as it occurs in this mixture has a low solubility in water . It is the unique properties of the mixture. Thus, there is no need for separation KCl from the aluminum in the mixture prior to applying the mixture as a soil fertilizer.
Slag and contains further metals which have value as micronutrients or secondary nutrients. These include magnesium, copper, manganese and zinc. As noted, nitrogen in aluminum nitride slowly converts to ammonia, which is a valuable plant nutrient in addition to its value in neutralizing any acid generated by the hydrolysis of chloride salts.
Example 1. It illustrates a typical application of the invention for the reclamation of aluminum from used beverage cans (UBC) potassium chloride (KCl) as a total replacement NaCl in the salt flux.
Apply a rotating drum oven with gas heating capacity of 15,000 pounds, which was charged with the following materials
The crushed body
3004 grade aluminum cans and a length and a width of 0.25-1.5 inches (0,6-3,8) 0,0045-0,011 cm and a thickness of inch (0,011-0,03 cm)
The ground cover
From aluminum cans mark 5042 \ 5182, the length and width of 0.25-1.5 inches (0,6-3,8 cm) and a thickness of 0,010-0,013 inches (0,025-0,033 cm)
The total load of 15,000 pounds, divided into three equal portions,
Generally KCl 3,400 pounds; parts of each portion used for beverage cans.
The furnace is initially cleaned with 500 pounds of KCl, then charged flux KCl, heated to about 100-200 o F (55-110 o C) higher than normal with an internal gas burner, and rotated for about 20 minutes. Then add the first batch of aluminum scrap and the mixture is rotated in the furnace for about 1500 rpm to mix its contents during scrap melting. The gas burner is switched off and removed from the furnace and the aluminum metal pool which had gathered at the furnace bottom, emptied. Then added Subsequent batches of aluminum scrap, using the remaining portions of the flux, for approximately the same times and at approximately the same temperature.
Aluminum pools extracted from the furnace amount to 85.0% of the scrap metal charged for the first batch and 90.0% of the scrap metal charged for the second and third parties.
Example 2. It shows a typical application of the invention for the reclamation of aluminum from scrap class I, which has not been decorated (printed without labels) and not in contact with the product.
The content of the following downloads
Aluminum scrap
L Class 15,000 pounds, divided into two batches of approximately equal size
KCl: 2,700 pounds total; portions for each scrap batch
loading sequence and operating conditions are the same as in Example 1. In this case, the amount of aluminum baths 94.5% of the scrap metal charged.
Example 3. It shows a typical application of the invention for aluminum scrap Class III. Class III scrap and scrap consists of aluminum body and skeleton, which has been decorated (printed matter applied) but has not been in contact with the product.
The content of the following downloads
Aluminum scrap
Class III 30,000 pounds, divided into three batches of approximately equal size
KCl: 2,800 pounds total, in portions for each scrap batch
loading sequence and operating conditions are the same as in Example 1. In this case, the aluminum bath from the three batches amount to 91.3%, 96.6% and 93.7%, respectively, of the scrap metal charged for those batches.
sodium sulfate, derived from heats implemented in ways similar to those used in Examples 1 to 3 were analyzed by ICAP. Analysis results for selected metals listed in Table. 1. Sodium sulphate present in represents residual sodium chloride left in the furnace from previous runs in which the flux is applied as sodium chloride. As can be seen, the sodium level listed in the Table. 1, decreased from a high level during the reclamation of aluminum from used beverage cans, in which the first melt was performed with flux, consisting of a 100% KCl to extract aluminum from scrap class III, which was the last of the three. This is a result of cleaning of the furnace rather than the presence of any sodium in the reclamation of aluminum.
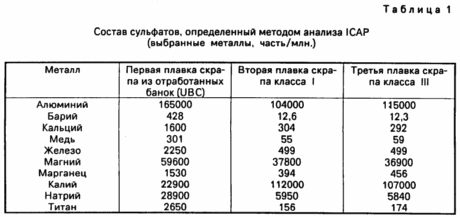
These data, together with the aluminum recovery data in Examples 1 and 2 show that KCl functions effectively as a flux in the absence.
Example 4. The solubility of sulfate components in water was determined according to the pH behavior as a measure of sulfate as a fertilizer for the soil. 3, which has a size in the range 0.15-3 mm - As described in Example 1 was used as a test sulfate typical sample taken from aluminum reduction methods.
To determine water solubility, the samples were added to deionized water adjusted to a selected pH in the range 0,6-5,6. Relationships were applied to 50 ml water with pH adjusted to 1 g of the granulated saltcake, shaken on a rotary shaker at a speed of 170 rev / min for 16 hours and filtered through a filter with a pore size of 2 microns. The final pH of the filtrate. To determine total concentrations of each component in the sulphate additional samples were boiled in HNO 3 and HClO 4.
Analysis for potassium and sodium were performed by atomic emission spectrometry, while analyzes of aluminum and magnesium were performed by atomic absorption spectrometry. The total concentration of the sample is listed in Table. 2 together with the pH of the filtrate obtained by exposing the sample to deionized water and filtering according to the method described.

The levels of these four metals in the filtrates, which are indications of the water solubility at different pH levels, are listed in Table. 3.
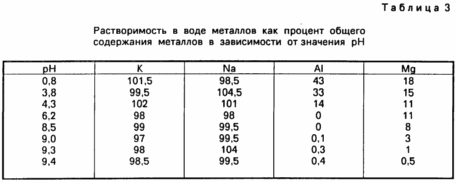
The data presented in the Table. 3 shows that solubility of sodium and potassium are independent of pH and that the sodium and potassium salts are totally water soluble over the entire pH range, wherein a deviation errors are allowable limit. Unlike sodium and potassium, and magnesium aluminum solubility depends on the pH, the solubility increases with decreasing pH. Under even the most highly acidic conditions (pH 0.8), the magnesium solubility reached only 18% of the total magnesium content, and the aluminum solubility reached only 43%
This solubility data indicates that the material can be used as soil fertilizer. The entire potassium content is soluble and therefore available for plant uptake, whereas the aluminum is of such low solubility that it increases slightly if there is any danger of phytotoxicity.
Example 5. This example illustrates the use of saltcake obtained in accordance with the invention as a soil fertilizer. As the test crop spring wheat was used, and during testing sulfate fertilizer compared to conventional 0-0-60 grade potassium fertilizers. Additional measurements were carried out on the measurement sulfate suitability for wheat, aluminum phytotoxicity and trace metal uptake. As it demonstrated and explained in detail and shown in the Table. April 10, the results were very positive. In these tables, the screened sample sulfate referred to as "By-product."
The saltcake sample used in these tests was screened to 0.15-3 mm in size, and analyzed for elemental content in the liquid phase by digestion in HNO 3 and HClO 4. Solid phase analyzes were conducted using X-ray diffraction system, and analysis of both the solid and liquid phases was performed using a polarized light microscope, Nikon Optofot.
As a soil sample taken in these tests silt loam soil Vinnivilla treated with lime to pH 6.5 to 7.6 by adding 0.8 and 3g lime per 1 kg of soil, respectively. Lime used was a mixture of CaCO 3, MgCO 3 = 4 1 applied to 200kg soil for each pH. After lime application, the soil was wet to field capacity level for the precipitation of lime in less than one week, then placed in pots with soil 8kg soil per pot. In each pot, nitrogen and phosphorus in the form of NH 4 NO 3 and cuperfosfat (TSP), respectively, wherein the quantity of nitrogen amounted to 225 mg / kg of nitrogen, phosphorus, 250 mg / kg. As the medium used micronutrient mixture borate and sulfate salts in an amount of 4.7 mg of Mg, 4,7 mg Fe, 3,7 mg Mn, 4,3 mg Zn, 1,2 mg Cu, 0,5 and 21 mg As, 7 mg S per kg of soil. Since the soil already contained a considerable amount of potassium, corn was grown in the soil to extract potassium and potassium reduce the content available, thereby to achieve increased susceptibility experiments results. This was achieved by growing two successive corn crops on the soil - eight corn plants per pot for 44 and 34 days. After receiving the first corn crop, supplemental N was administered as a solution NH 4 NO 3 62.5 mg of the additive per 1 kg of nitrogen soil.
Quantities of extractable potassium in the soil determined extractant Mehlich, which contained 0,025 NH 2 SO 4 and 0,05N HCl. This was achieved by adding a 12.5kg soil sample to 50 ml of extractant Mehlich, shaking the mixture on a rotary mixer for 5 minutes, then filtering. The potassium in the filtrate was analyzed by atomic emission spectroscopy. The amount of extractable potassium in the soil determined in this manner was 63mg / kg and 68mg / kg at soil pH's of 6.5 and 7.6 respectively, before growing culture. After removing the contents of the first crop of wheat extractable potassium decreased to 11mg / kg and 22mg / kg respectively. After the second crop these values decreased further, to 5mg / kg and 15 mg / kg and a pH of 5.5 and 7.2 respectively.
After the cultivation, the soil removed from the pots and homogenized wheat for each pH. To continue testing of wheat, 1 kg of mixed soil with 0.73g of sand for each pot. Sand was added to reduce the potassium content and maximizing the volume of the medium used for the growth of plants. The nutrients N, P and S were added to the pots in an amount of 280 mg / pot nitrogen (in the form of added NH 3 MO 4), 120 mg / pot phosphorus (in the form TSR) and 23.6 mg / pot for S (in the form of CaSO 4 ).
In some pots added particulate sulphate as defined above, as a source of potassium, whereas in other pots was not added 0-0-60 grade potash fertilizer (based on potassium hydroxide). The latter contained 552.3% K as determined by acid digestion and atomic emission spectroscopy. Soil mixture in each pot were thoroughly mixed and prepared in an identical way to a control sample which was not added potassium source.
In each pot were planted fifteen seeds of Thatcher spring wheat at a depth of 0.5 inches (12.7 mm). After 6 days, the plants in each pot proryadili to 8 plants. Plants were grown under lamps of radiating high power, and, when required, water was added to maintain the water level in each pot of 230 g / kg dry soil. The maximum daily temperature was 85 to 75 o F (23,89 28,89 o C), and the minimum temperature of 65 o F (15,56 21,11 o C) .
After 42 days after planting, the plants were harvested stem. The roots were collected by separating them from the soil and washing them in 0.05M HNO 3. The stems and roots were dried in a forced air circulation, weighed and ground for chemical analyzes. The soil was air dried, sieved through a sieve with a mesh size of 2 cm and placed in plastic containers for storage prior to chemical analysis.
The stems and roots were digested by dry ashing for 6 hours at a temperature of 475 o C, dissolving in 2 N HNO 3 and filtering. The filtrates from portions were analyzed for K, Al, P, Ca, Mg, Ti, Fe, Zn, Pb, Cu, Ni and Cr, the roots and the filtrates were analyzed for K and Al. Methods of analysis included atomic emission spectroscopy for K and Ti, colorimetry for P, atomic absorption spectroscopy for Al, Ca, Mg, Fe, Zn, Pb, Cu, Ni and Cr and a method of Kjeldahl (modification thiosulfate salicylic acid) to total nitrogen. Absorption plant nutrients or trace metals was calculated by multiplying dry matter weight of plant root or the concentration of nutrients or trace metals in these parts of the plant.
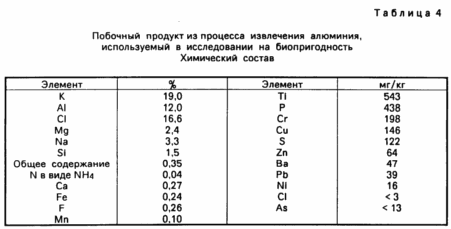
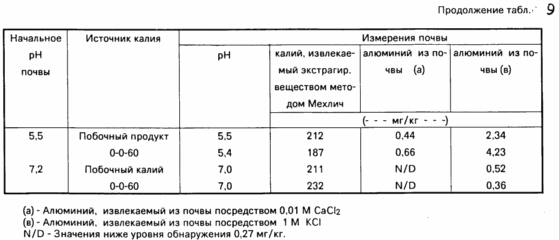
Besides Mehlich method for determining extractable potassium content was measured and the content of extractable potassium alternately by 0.01M CaCl2 and 1M KCl. A method for determining using CaCl 2 gives indication aluminum content available to plants, whereas the KCl method indicates exchangeable aluminum content. For the method using CaCl 2 20 ml of CaCl 2 solution was added to 10 mg of the soil, shaken for five minutes, then filtered. For the KCl method, 50 ml of KCl solution was added to 5g of the soil, shaken for 30 minutes, then filtered. The aluminum content is determined in the filtrates by a colorimetric method using 8-hydroxyquinoline. sulphate Ingredients tab. 4, and the results of various experiments are shown in Table. May 10.
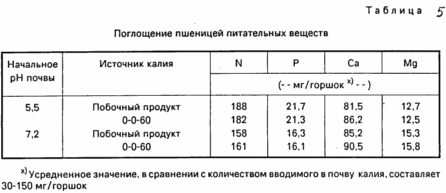
Table. 5 shows that the absorption of nitrogen, potassium and magnesium stalks of wheat affects neither the pH value of the soil or the amount administered potassium or potassium source.
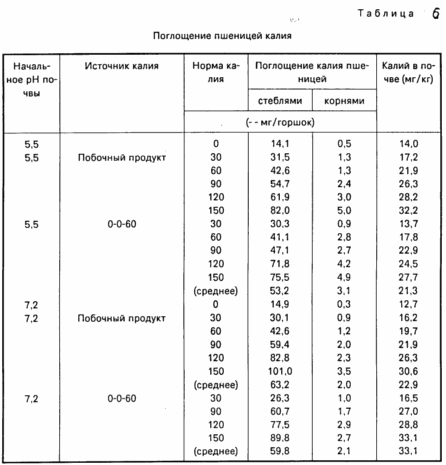
Table. 6 shows that the uptake of potassium stems and roots of wheat was the same regardless of the K source, when present in the soil extractable potassium Mehluch I. Although the difference seems, lies in the connection between the values of extractable potassium and Mehlich I soil pH between the two K sources, however, the presence of potassium in potassium absorption values and potassium Mehlich I had the same soil.
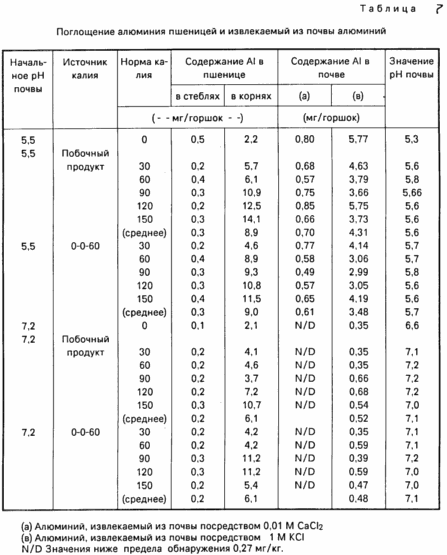
aluminum uptake study shown in Table. 7 shows that there are no differences between the two sources of potassium, which means that no aluminum sulphate bioavailability. This is true for recoverable aluminum in the soil.
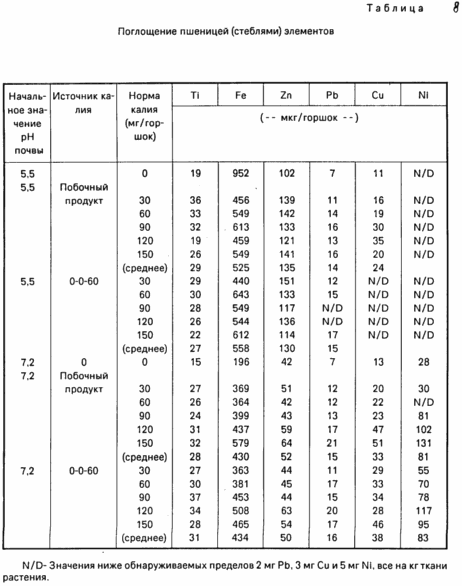
and absorbance elements results of these measurements are presented in Table. 8. Here again, it is seen that there is no significant difference between the two sources of potassium.
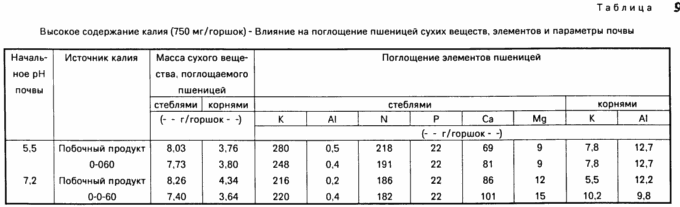
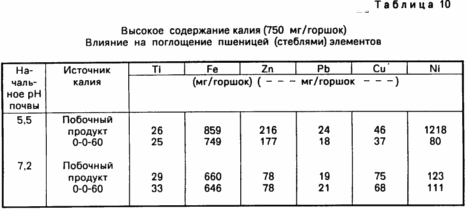
To investigate potential hazards from a large number of ground potassium or permanent and repeated applications over a time interval tests were conducted using a high content of potassium, for example 750 mg / pot. The results are shown in Table. 9 and 10. The data in the Table. 9 show that there is no difference in root dry matter weight basis or potassium aluminum absorption rate and plant stems and roots between the two sources. The same is true of plants absorbing elements, as indicated in Table. 10, especially at a pH of 7.2.
 |
 |
 |
Overall, the data indicate that the use of a by-product of aluminum extraction process as a potassium fertilizer is acceptable from the point of view of Agronomy. The potassium in the by-product and the potassium in conventional 0-0-60 grade potash fertilizer is equally water-soluble and bioavailable, and there is no evidence of enhanced bioavailability of by-product aluminum, despite the fact that the by-product contains 12% Al. Nor is there any evidence of increased availability of elements.
Slag, formed from the aluminum reduction process, resulting in the application of KCl flux in accordance with this invention, such as that described in the examples may be further processed by any of the various ways in order to be suitable for use as fertilizer. The material can be used in a variety of solid fertilizer, and the methods of processing the material, particle size, the presence and amount of additional ingredients, and other parameters are possible in the final application to the fertilizer into the soil, will vary depending on the particular mode of fertilizer into the soil by type of crop, type and location of the field, where the fertilizer will be made.
For most applications, the dross will first be comminuted to particles, generally of about 10mm diameter or less and preferably 5 mm or less depending on the final shape. Among the various types of forms, which may take a by-product for use as a fertilizer, can be suspensions, powders, granules and tablets.
To simplify the distribution or in order to modify the physical properties of the by-product used as a fertilizer, can contain additives. Examples of such additives are carriers, diluents, anti-caking agents, and conditioners of various kinds. These additives may include clays, gels, diatomaceous earth, vermiculite, wetting agents, humectants, organic matter such as comminuted corn cobs, and a many other such additives known in the fertilizer applied. The by-product and can be supplemented with additional nutrients to achieve desired combinations of different types of nutrients.
After cooking by-product for use as a fertilizer can be introduced into the soil in the conventional manner. Fertilization methods include spraying the plane and enter the soil to ground level, applying methods such as, for example, dusting, sprinkling, scattering during harrowing the soil, mixing, or adding the composition to the water for irrigation.
CLAIM
1. A method for fertilizing soil comprising amendment of the nutrient mineral mixture consisting of potassium salts and trace elements, characterized in that the mixture is used as the aluminum-containing nutrient waste pretreated with molten flux and subsequent separation of the aluminum salt phase while the salt phase is crushed to a particle size not exceeding 10 mm.
2. The method of claim. 1, characterized in that the aluminum waste containing alumina, salts, oxides or nitrides of barium, calcium, copper, iron, magnesium, manganese and titanium, and aluminum nitride.
3. The method of claim. 1 and 2, wherein the molten flux is used containing 90 95 wt. potassium chloride.
4. The fertilizer for soil nutrient mixture comprising a potassium salt, characterized in that the nutrient mixture as it comprises aluminum waste pretreated with molten flux and subsequent separation of the aluminum salt phase while the salt phase is crushed to a particle size not exceeding 10 mm.
5. The fertilizer of Claim soil. 4, wherein the waste contains aluminum oxide, aluminum salts, oxides or nitrides of barium, calcium, copper, iron, magnesium, manganese and titanium, and aluminum nitride.
6. The fertilizer to the soil according to claims. 4 and 5, wherein the molten flux 90 containing 95 wt. potassium chloride.
print version
Publication date 04.03.2007gg





Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.