| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2284184
![]()
METHOD OF TREATMENT OF CANCER OF THE VACCINE AND STOMACH
The name of the inventor: EJENIE Jefferhazen A. (US); BENNER Stephen E. (US); DAGEN Terry S. (US)
The name of the patent holder: BRISTOL-MAERS SCWIBB COMPANY (US)
Address for correspondence: 119034, Moscow, Prechistenskiy per., 14, p. 1, 4th floor, "Gowling International Inc.", Y.V. Dementieva
The effective date of the patent: 2002.03.04
The invention provides a method for treating esophageal cancer, or cancer of the esophagus-to-stomach connection region, or gastric cancer in humans. The method comprises orally administering a combination of tegafur and uracil at a tegafur dose of 100-500 mg / m 2 / day in a mole ratio of tegafur and uracil 1: 4; Oral administration of 0.1-500 mg / kg / day of folinic acid or a pharmaceutically acceptable salt thereof; Intravenous administration of paclitaxel and carboplatin at a dose of 10-300 mg / m 2 and 100-500 mg / m 2, respectively, with paclitaxel and carboplatin being administered on day 1 of the 28-day cycle, and tegafur, uracil and folinic acid or a pharmaceutically acceptable Salt - in the 2-22 days. And the 23-28th days of the cycle are a break. In this case, the treatment regimen is repeated at least once. The invention provides effective cancer treatment and good tolerability by increasing the activity of paclitaxel and carboplatin when combined with the listed preparations, and a more gentle administration of chemotherapeutic agents compared to known routes of administration.
DESCRIPTION OF THE INVENTION
The present invention provides the administration to a warm-blooded animal of a combination of tegafur, uracil, folinic acid, paclitaxel and carboplatin for the treatment of tumors.
5-fluorouracil (5-FU) is a known anti-tumor agent. The combination of 5-fluorouracil and folinic acid is known for the treatment of colorectal cancer. Tegafur (1- (2-tetrahydrofuryl) -5-fluorouracil) is a prodrug of 5-fluorouracil. In vivo, 5-fluorouracil is rapidly inactivated by the enzyme dihydropyridine dehydrogenase (DPD). Uracil inhibits the DPD metabolism of 5-FU formed from tegafur. Thus, the combined administration of uracil and tegafur results in a longer-lasting action of active 5-FU compared to one tegafur. It is known that 5-fluorouracil can not be administered orally.
US Pat. No. 4,388,229 discloses an anticancer composition comprising 1- (2-tetrahydrofuryl) -5-fluorouracil ("tegafur") and uracil. The composition is used to deliver 5-fluorouracil to a tumor sensitive to 5-fluorouracil in warm-blooded animals. It is indicated that the composition can be administered in various dosage forms, including an oral dosage form.
US Pat. No. 5,543,513 discloses an antitumor composition containing tegafur and uracil in a molar ratio of 1: 4. It is claimed that this antitumor composition may become more active when administered with folinic acid or a pharmaceutically acceptable salt thereof. This patent states that the described combination can be administered in various dosage forms, including oral.
Paclitaxel (TAXOL®), a taxane diterpene, is a natural substance isolated from the Pacific yew bark, Taxus brevifolia. Studies have shown that it has excellent antitumor activity against a set of tumors in in vivo animal models, including, for example, breast and ovarian tumors. Paclitaxel is an antimitotic agent that mainly binds to microtubules. Stabilization of microtubules with paclitaxel inhibits the restructuring of the microtubule network. Paclitaxel is usually administered intravenously or by infusion.
Carboplatin (PARAPLATIN®) is a known anti-tumor agent that induces DNA cross-linking associated with proteins and non-proteins. This effect is nonspecific for the cell cycle. Carboplatin is usually administered by intravenous infusion or injection.
Applicants have found that 5-fluorouracil can increase the activity of paclitaxel and carboplatin. However, due to the fact that 5-fluorouracil can not be administered orally, this combination has to be administered less gently, for example by intravenous injection, and this usually requires the availability of qualified medical personnel.
It would be highly desirable to provide such a method of treating tumors, especially esophageal tumors, the esophagus-stomach and stomach connection region, which would involve intravenous administration of paclitaxel and carboplatin and oral administration of 5-fluorouracil to a warm-blooded animal to effectively treat such tumors.
SUMMARY OF THE INVENTION
The present invention relates to a method for treating esophageal cancer, or cancer of the esophagus-to-stomach connection region, or gastric cancer in humans. The method comprises administering orally a combination of tegafur and uracil at a tegafur dose of 100-500 mg / m 2 / day in a mole ratio of tegafur and uracil 1: 4; Orally 0.1-500 mg / kg / day of folinic acid or a pharmaceutically acceptable salt thereof; Paclitaxel and carboplatin intravenously at a dose of 10-300 mg / m 2 and 100-500 mg / m 2, respectively, with paclitaxel and carboplatin being administered on day 1 of the 28-day cycle, and tegafur, uracil and folinic acid or a pharmaceutically acceptable salt thereof - in the 2-22 days, and the 23-28th day of the cycle is a break.
If necessary, the treatment regimen is repeated at least once.
DETAILED DESCRIPTION OF THE INVENTION
The combination of tegafur and uracil in amounts sufficient to convert tegafur to 5-fluorouracil (preferably, the molar ratio is about 1: 4). May be administered orally. It was found that with the oral administration of this combination, a sufficient amount of 5-fluorouracil is formed, and along with paclitaxel and carboplatin, active and effective treatment of tumors, especially those, is provided. Which are associated with tumors in the esophagus, in the area of the esophagus with the stomach and in the stomach.
In one oral dosage form according to this invention, tegafur, uracil and folinic acid, preferably in the form of a calcium folate calcium salt, are in one dosage unit form. Otherwise, and preferably tegafur and uracil are in the first oral dosage form, and folinic acid, preferably in the form of calcium folinate, is contained in the second oral dosage form. The dose of each active ingredient administered per day is about 0.1-100 mg / kg / day, preferably about 1-30 mg / kg / day, for tegafur. Uracil is administered at a dose of about 1-50 mg / kg / day. The UFT dose, namely the 1: 4 combination of tegafur and uracil, is about 100-500 mg / m / day, calculated on tegafur, preferably about 200-300 mg / m 2 / day, calculated as tegafur. Folinic acid or a pharmaceutically acceptable salt thereof can be administered in an amount of about 0.1-500 mg / kg / day, but it is preferably administered as calcium folinate at a fixed dose of about 90 mg / day. The oral dosage form (s) can be administered in a single dose or in divided doses, usually up to 3 times a day.
Paclitaxel and carboplatin are preferably administered each non-orally, it is better to administer them by intravenous infusion. Depending on the surface area of the body, the infusion dose of paclitaxel may range from about 10 to 300 mg / m 2 , preferably about 30 to 200 mg / m 2, and more preferably about 100, 135 or 175 mg / m 2 . Before the infusion of paclitaxel, a preliminary treatment known to specialists should be performed. The dose of paclitaxel is preferably administered intravenously by infusion for at least about 3 hours, preferably for about 3 or 24 hours. The dosage of carboplatin is preferably administered intravenously by infusion, preferably for at least about 15 minutes. The infusion dose of carboplatin may be about 100-500 mg / m 2 , preferably about 300-360 mg / m 2 . It is also possible to calculate the infusion dose of carboplatin according to the Calvert formula in the form of AUC, approximately 4-6 mg / ml · min.
Those skilled in the art will be able to determine the above doses of UFT, folinic acid or a pharmaceutically acceptable salt thereof, paclitaxel, calculated for example on the surface area of the body and / or taking into account the toxicity and carboplatin according to the Calvert formula, as described below. According to the present invention, a combination of tegafur and uracil (eg, UFT) provides a sufficient amount of 5-fluorouracil in combination with paclitaxel and carboplatin to achieve effective treatment of tumors, especially esophageal tumors, the esophagus-stomach and gastric junction region, by a gentle method.
According to a preferred embodiment, the present invention provides a method of treating cancer, particularly cancerous tumors in a warm-blooded mammal, which comprises administering the active agents according to a scheme generally based on a 28-day cycle. For example, paclitaxel at a dosage of about 100, 135 or 175 mg / m 2 , preferably about 175 mg / m 2 , and carboplatin at a dose of about 300-360 mg / m 2, or at a dose corresponding to the desired area under the concentration curve (AUC) , 4-6 mg / ml min, preferably about 6 mg / ml min, as determined by the Calvert formula, each may be administered on day 1 of the 28-day cycle, and UFT at a dose of about 200, 250 or 300 mg / m 2 / day counting on tegafur and calcium folinate at a dose of approximately 90 mg / day can be administered daily for the 2nd-22nd day, and no active agents are administered on day 23-28. If necessary, repeat the 28-day cycle. The dose of carboplatin is calculated before each course of therapy according to the Calvert formula:
Carboplatin in mg (total dose) = (desired AUC) × (GFR + 25)
The required AUC is approximately 4-6 mg / ml · min. The glomerular filtration rate (GFR in ml / min) is approximated by measuring the clearance of the creatinine (Cr. Cl.) Of the patient, which is calculated according to the patient's age (years), weight (kg) and serum creatinine (mg / dl) Crockroth-Gaulta:
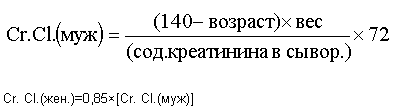
Dosage forms can be prepared using, for example, conventional solid or liquid carriers or diluents, and pharmaceutical additives depending on the method of administration (for example, excipients, binders, preservatives, stabilizers, flavors, etc.), methods known in the art Pharmacy.
Dosage forms for oral administration include a tablet, powders, granules and the like. Excipients and additives that can be used include, but are not limited to, lactose, sucrose, sodium chloride, glucose, urea, starch, calcium, kaolin, crystalline cellulose, salicylic acid, methylcellulose, glycerin, sodium alginate, resin and the like. Conventional binders can be used, for example glucose solutions, starch solutions, gelatin solutions, and the like. Disintegrating agents include, but are not limited to, dry starch, sodium alginate, agar powder, calcium carbonate and the like. Absorbents may include, but are not limited to, starch, lactose, kaolin, bentonite and the like. Lubricants include, but are not limited to, purified talc, stearic acid salts, boric acid powder, polyethylene glycol, and the like.
Dosage forms for parenteral administration, for example subcutaneously, intravenously, intramuscularly, or by injection into the sternum or by infusion, include injectable solutions or suspensions which may contain, for example, pharmaceutically acceptable diluents or solvents, for example mannitol, 1,3-butanediol, water, Ringer's solution, isotonic sodium chloride solution or other suitable dispersants or wetting and suspending agents, including synthetic mono- or diglycerides, and fatty acids, including oleic acid.
The following examples illustrate the invention, but are not intended to limit it.
Example 1
This study assesses the in vivo dose-limiting toxicity of UFT (tegafur and uracil at a molar ratio of 1: 4) in combination with calcium folate at 3 times a day for 21 days and in combination with TAXOL® (single dose, infusion over a 3- (One dose, infusion for 1 hour on the 1st day of the cycle) in patients with esophageal, gastric or esophageal connective with the stomach and allows to determine the recommended dose used In Phase II of the study.
The standard I phase was performed with increasing doses of UFT at a fixed dose of folinatal calcium (leucovorin) equal to 90 mg / day, a fixed dose of TAXOL® of 175 mg / m 2 administered by infusion for 3 hours on day 1, And a dose of PARAPLATIN® corresponding to the required area under the curve (AUC) of about 6 mg / ml · min calculated by the Calvert formula administered by infusion for 1 hour. The initial dose of UFT was 200 mg / m 2 / day, calculated on tegafur, was administered with leucovorin at a dose of 90 mg / day, the doses of both agents being divided into 3 doses and injected for 21 days starting on day 2, after which Followed by a 6-day break.
Doses of UFT were increased in groups of 3-6 patients. The dose and schedule of leucovorin administration remained unchanged. An increase in the UFT dose was carried out until ![]() 1 of the first 3 patients or
1 of the first 3 patients or ![]() 2 out of 6 patients did not begin to experience toxicity (the maximum permissible dose limited by toxicity). The next immediately reduced dose, the maximum tolerated dose (MTD), is recommended for Phase II of the experiment.
2 out of 6 patients did not begin to experience toxicity (the maximum permissible dose limited by toxicity). The next immediately reduced dose, the maximum tolerated dose (MTD), is recommended for Phase II of the experiment.
Groups of at least 3 patients received the following doses of UFT: 200 (DL200), 250 (DL250) or 300 (DL300) mg / m 2 / day, calculated on tegafur.
The criteria for the study include, but are not limited to, histologically or cytologically confirmed metastatic or inoperable local squamous carcinomas or adenocarcinomas of the esophagus, or areas of the esophagus with the stomach, or gastric adenocarcinoma, radiation therapy has not been performed concurrently, chemotherapy for metformin tumors has not been performed. ECOG 0- 2, there was no metastasis in the brain and the corresponding violations of hematologic, renal and hepatic functions.
Treatment was conducted for 4 weeks until the development of the disease or unacceptable toxicity began to occur. TAXOL® was infused for 3 hours, followed by the administration of PARAPLATIN® intravenously at a dose corresponding to the surface area under the curve (AUC) of approximately 6 mg / ml · min, determined by the Calvert formula on the 1st day of each cycle. Then, UFT and leucovorin were administered orally on the 2-22 days of each cycle followed by a 6-day break. The initial dose of UFT was 200 mg / m 2 / day, calculated on tegafur, was administered daily in three portions. The dose of leucovorin was fixed and equal to 90 mg / day, taken in three portions simultaneously with UFT at 8-hour intervals. Cycles of treatment were repeated every 28 days.
The dose of PARAPLATIN® was calculated before each course of therapy for each patient according to the following procedure. We used the Calvert formula:
PARAPLATIN® in mg = (required AUC) × (GFR + 25)
The required AUC is approximately 6 mg / ml · min. The rate of glomerumeric filtration rate (GFR in ml / min) was approximated by measuring the clearance of creatinine (Cr. Cl.) Of the patient, which was determined from age (years), weight in kg and serum creatinine level in mg / dL in a patient according to the Crockroth formula -Gaulta:
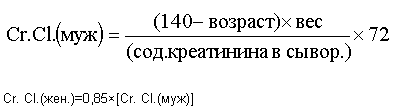
After all patients safely underwent one treatment cycle, the dose of UFT was increased. The study was continued to each progressing level until the maximum tolerated dose (MTD) was reached. MTD was defined as the dose value at which more than 1/3 or 2/6 of patients experienced dose-limiting toxicity (DLT) during the first treatment cycle.
The DLT was determined as follows:
Hematologic Toxicity:
- Neutropenia 3/4 degree, complicated by concomitant fever, or
- Thrombocytopenia 3/4 degree, complicated by bleeding or requiring platelet transfusion, or
- Thrombocytopenia 4 degrees.
Non-hematological toxicity:
- Nausea more than 3 degrees, vomiting, diarrhea despite medical intervention, or
- Another type of non-hematologic toxicity of more than 3 degrees, or
- Inability to receive
 75% of the planned dose of UFT / leucovorin or
75% of the planned dose of UFT / leucovorin or - The delay of the beginning of the next cycle is more than 2 weeks.
Patients were included in the protocol if they met the following criteria:
- Histologically or cytologically confirmed metastatic or inoperable local squamous carcinoma or adenocarcinoma of the esophagus, or areas of the esophagus with the stomach, or adenocarcinoma of the stomach;
- Measurable (> 1.5 cm in both dimensions) or a noticeable disease (
 1.5 cm in at least one dimension);
1.5 cm in at least one dimension); - Characterized by adequate hematologic, hepatic and renal function;
- Age> 18 years;
- Until then, they had not been subjected to chemotherapy or immunotherapy, including ancillary and non-adjuvant treatment;
- ECOG status 0-2 (on the Zubrod scale), life expectancy> 3 months;
- written agreement.
Patients were excluded if they had an intestinal obstruction or any condition interfering with the absorption of UFT and / or leucovorin, or there were previous radiotherapy sessions, unless they were associated with palliative or adjunctive treatment of metastatic or local malignant adenocarcinoma or squamous esophageal carcinoma, the area Connect the esophagus with the stomach or stomach.
In the first phase of the experiment, 16 patients participated. Toxicity limiting the dose was observed during cycle 1 in two patients. With DL200, one patient experienced grade 3 nausea and vomiting, resulting in a loss of 32% of UFT doses, with DL300 one patient experienced grade 3 myalgia. DL300 was considered a tolerable dose, and it was given to 9 patients. During cycle 2, adverse effects associated with DLT were observed in one patient with a neutropenia fever corresponding to more than 14 days of platelet recovery delay in one patient with grade 3 fatigue and one patient with grade 3 vomiting. In all cycles, other adverse symptoms ![]() 3 degrees included fever with neutropenia, neuropathy, deep venous thrombosis, chills, vomiting, constipation and fatigue. Clinical responses included: partial responses were observed in 6 patients who typically had a tumor reduction of at least 50%; Stable disease was observed in 4 patients when there was no change in the disease (namely, a decrease in the tumor size by less than 50% or an increase in the tumor size by less than 25%). Progression of the disease was noted in 5 patients. This group had new, previously undetected pathological changes, or malignant pleural efflux or ascites appeared and / or increased at least 25% in the size of one or several notable lesions. The treatment regimen described above was usually well tolerated and showed antitumor activity at all doses. One patient could not determine the answer.
3 degrees included fever with neutropenia, neuropathy, deep venous thrombosis, chills, vomiting, constipation and fatigue. Clinical responses included: partial responses were observed in 6 patients who typically had a tumor reduction of at least 50%; Stable disease was observed in 4 patients when there was no change in the disease (namely, a decrease in the tumor size by less than 50% or an increase in the tumor size by less than 25%). Progression of the disease was noted in 5 patients. This group had new, previously undetected pathological changes, or malignant pleural efflux or ascites appeared and / or increased at least 25% in the size of one or several notable lesions. The treatment regimen described above was usually well tolerated and showed antitumor activity at all doses. One patient could not determine the answer.
CLAIM
A method of treating esophageal cancer or cancer of the esophagus or stomach cancer region in humans, comprising administering an oral combination of tegafur and uracil at a tegafur dose of 100-500 mg / m 2 / day in a mole ratio of tegafur and uracil 1: 4; Orally 0.1-500 mg / kg / day of folinic acid or a pharmaceutically acceptable salt thereof; Paclitaxel and carboplatin intravenously at a dose of 10-300 mg / m 2 and 100-500 mg / m 2, respectively, with paclitaxel and carboplatin being administered on day 1 of the 28-day cycle, and tegafur, uracil and folinic acid or a pharmaceutically acceptable salt thereof - in the 2-22 days, and the 23-28th day of the cycle is a break.
The method of claim 1, wherein the treatment regimen is repeated at least once.
print version
Date of publication 27.11.2006gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.