| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2200954
![]()
METHOD FOR PREDICTING THE PECULIARITIES OF CURRENT HEPATITIS B
IN PATIENTS WITH HEMOBLASTICS
The name of the inventor: Ovsyannikova EG; Zaklyakova L.V.
The name of the patent holder: Ovsyannikova Elena Georgievna
Address for correspondence: 414000, Astrakhan, ul. Baku, 121, AGMA, patent engineer SA Golubkina
Date of commencement of the patent: 2001.01.09
The invention relates to the field of medicine, in particular to gastroenterology and hematology. The method provides an increase in the accuracy of predicting the features of the course of chronic hepatitis B in patients with hemoblastosis. An immunogenetic study is carried out, while the antigens of the loci A, B, C of the HLA system are determined in venous blood and chronic Hepatitis B and hemoblastosis are predicted in the presence of the HLA-B35 antigen; in the presence of the HLA-B40 antigen, chronic hepatitis B is predicted, in the presence of an A2 / B27 haplotype Predict a resistance to infection with the hepatitis B virus.
DESCRIPTION OF THE INVENTION
The invention relates to the field of medicine, namely gastroenterology and hematology, and can be used, in particular, for predicting the features of the course of chronic hepatitis B (CHB) in patients with hemoblastosis.
From the practice of medicine, only one method for predicting chronic hepatitis B is known (Sochnev AM, Alekseev LP, Tananov AT Antigens of the HLA system for various diseases and transplantation .- Riga: Zinatze, 1987 - 86 seconds.) , Consisting in the determination of the antigenic profile of the HLA system.
The disadvantage of this method is that the method is known only in patients with chronic diffuse liver diseases (CDD). Patients with hemoblastosis with chronic hepatitis B have significant differences from patients with CDD (immunosuppression, many blood transfusions, massive drug loading) and apply the known method of predicting CHB, it is not possible.
The aim of the proposed invention is to increase the accuracy of predicting the features of the course of chronic hepatitis B in patients with hemoblastosis.
The aim of the invention is achieved in that the antigens of the loci A, B, C of the HLA system are determined in venous blood and predict chronic hepatitis B and hemoblastosis in the presence of HLA-B35 antigen; In the presence of HLA-B40 antigen, chronic hepatitis B is predicted; In the presence of haplotype A2 / B27, resistance to infection with the hepatitis B virus is predicted.
Advances in the diagnosis and treatment of viral hepatitis achieved over the past decade have shown the importance of early diagnosis, the possibility of curing and predicting outcomes of hepatitis.
Predicting the nature of the course and outcome of disease is an important area of modern medicine. One of the reliable ways to determine the predisposition and characteristics of the course of the disease is the study of antigens of the HLA system.
Patients with hemoblastosis are a special group of patients for whom the problem of predicting the course of the characteristics of chronic hepatitis goes to one of the leading places. Chronic hepatitis B in patients with hemoblastosis is often layered on the already existing specific and medicinal liver damage. Early diagnosis of hepatitis B is hampered by the lack of vivid clinical symptoms, the main clinical and laboratory syndromes of chronic hepatitis B are overlapped by the leukemia clinic. The state of immunosuppression contributes to the development of latent subclinical forms of chronic hepatitis B. At the same time, the risk of contracting the hepatitis B virus in patients with hemoblastosis is large enough due to massive blood transfusion load and a variety of invasive interventions. Thus, this gives grounds for applying a method for predicting the features of the course of chronic hepatitis B in hemoblastoses.
The presented method tested 106 patients with hemoblastosis on the basis of the hematological department of the Aleksandro-Mariinsky I Regional Clinical Hospital of Astrakhan in the period from 1996 to 2000.
When processing the results, methods of medical statistics were applied. To evaluate the results of typing antigens HLA, the frequencies of antigens were calculated, based on the Hardy-Weinberg law. To determine the reliability of the results, a nonparametric criterion, X 2, was used .
The criterion X 2 was calculated using the Holdene formula, taking into account the small sample size, a modified formula was applied with the Yates correction. Values of X 2 exceeding 3.841 (corresponding to p <0.05) were considered as an indicator of the reliable difference between the frequencies in the compared groups. To improve the accuracy of the study, the value of "p" was corrected according to the Bonferroni method (ie multiplying the "p" value by the number of antigens).
In addition to the statistical significance, the association strength indicator RR (relative risk) was used. The value of RR, equal to 1, indicates the absence of association. RR> 1 (positive association), means that the antigen is more common in patients; If RR <1, this indicates a decrease in antigen frequency in patients. When calculating RR, taking into account the small sample size, a modified formula for small samples was applied.
The frequencies of HLA haplotypes were calculated by an indirect method, using the coefficient of nonequilibrium coupling by the Matthuis formula.
All calculations were carried out on a personal computer IBM Pentium-100, using standard statistical programs.
106 patients with hematological malignancies of Russian nationality who were on treatment in the hematological department of the I OKB underwent a complete clinical laboratory and instrumental examination for revealing signs of liver damage with hepatitis B. The group was formed using random selection, the study included patients with signs of liver damage (Increased levels of ALT, bilirubin, hepatomegaly), and patients who did not have these symptoms. All patients in this group were typed with HLA antigens by complement-dependent cytotoxicity. Control group - 200 healthy donors of Russian nationality in Astrakhan region and 30 patients with Russian nationality of HBsAg + CDD.
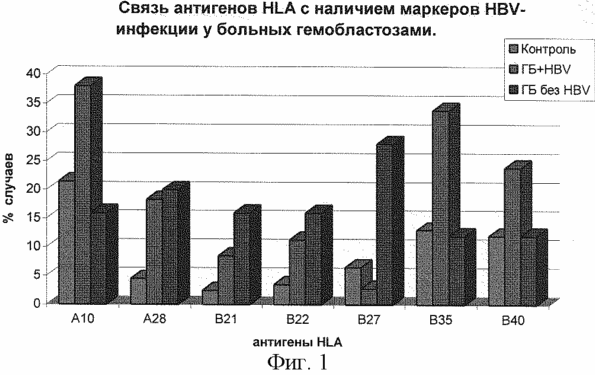
In Fig. 1 presents a comparative analysis of the difference in the frequency of HLA antigen detection in patients with hemoblastosis with markers of HBV infection and without markers. As a result of the analysis, it was established that in patients with hemoblastosis having markers of HBV, there is a significant increase in antigens HLA-A10, A28, B21, B22, B35, B40. Antigen-HLA-B27: significantly elevated in patients with hemoblastosis without HBV. Since the patients of this group have two main diseases - hemoblastosis and viral hepatitis B, we have attempted to determine which disease is labeling these antigens or the ability of one antigen HLA described by many authors to be a marker of several diseases. We analyzed the distribution of HLA antigens in patients with HBsAg + CDD and in hemoblastosis patients with HBV markers.
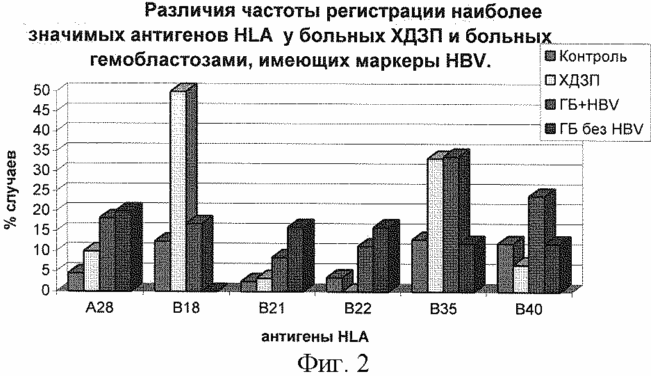
As shown in FIG. 2, the highest detection rate in both groups is HLA-B35 antigen. The HLA-B18 antigen was detected only in the HDZ group. HLA-B40 antigen only in the hemoblastosis group plus HBV infection. The analysis revealed that the antigens HLA-B35, B40 label the risk of HBV infection in patients with hemoblastosis. For a deeper analysis of this statement and detection of clinical and diagnostic criteria for hepatitis B virus in patients with hemoblastosis, we performed a comparative analysis of the distribution of HLA antigens in these patients with CVH and patients with HBsAb alone.
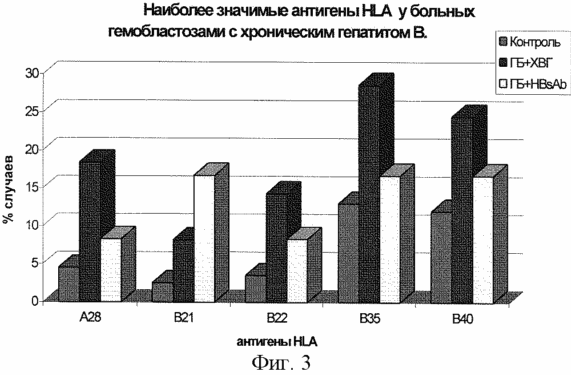
In Fig. 3, the greatest differences were revealed in the antigens HLA-B35, B40: there is a significant increase in the frequency of registration of these antigens in the group of patients with hemoblastosis plus chronic viral hepatitis B and the absence of a significant difference in the group of patients with hemoblastosis who underwent viral hepatitis B. There is a significant increase in the frequency of registration Antigen HLA-A28, B22 in patients with hemoblastosis with chronic hepatitis B and there is no significant increase in the percentage of detection of this antigen in patients with viral hepatitis B. We have thus discovered that CHB in patients with hemoblastosis develops in the presence of antigens HLA-A28, B22, B35 , B40.
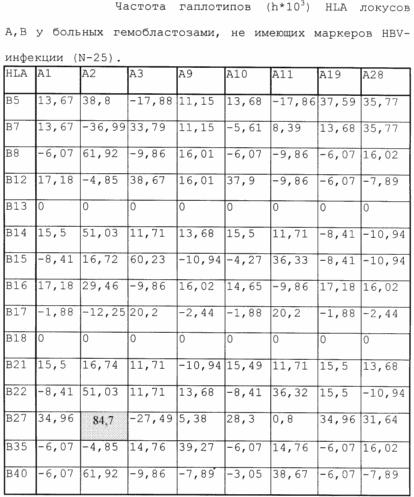
To identify more subtle differences between groups of patients studied, differences in haplotype HLA were analyzed.
From the table it follows that the resistance to HBV infection in patients with hemoblastosis is marked by haplotype A2 / B27 (the frequency of the haplotype is H = 84.7).
Below are the results of approbation.
Example 1
Patient G. of the history of the disease 1421. Entered the department on 30.01.97 with complaints of weakness, malaise, fever, pain in the bones, heaviness in the right hypochondrium. At the examination, anemia is revealed, which requires clarification. Typing of antigens of the HLA I class system of loci A, B, C was carried out with the help of the lymphocytotoxic reaction proposed in 1964 by Terasaki.
The reaction is based on a two-stage effect on peripheral blood lymphocytes: 1 - immune serum containing antibodies of known specificity; 2 - complement. To identify antigens HLA, sets of anti-leukocyte sera were used to antigene class I antigens (panels N 36 and N 37 for loci A, B, C) and complementary rabbits lyophilized. The reaction was carried out with the help of special micro equipment: Hamilton micro-syringes with a volume of 25 microliters with a mechanical dispenser; Tablet Terasaki for immunological reactions; Plastic tubes with a volume of 1 and 2 milliliters. To carry out the reaction, a preliminary digestion was carried out for Vaseline oil in the wells of the Terasaki tablet, 5 microliters of antisera. The plates thus prepared were frozen and stored until use in a freezer at -20 ° C. Immediately prior to the reaction, the sera were thawed at room temperature. During the reaction, a verophagin-fikol solution and a dye-5% eosin solution were used. Verografine-phycolite gradient was prepared as follows: 9% aqueous (distilled water) ficol solution, molecular weight 400,000 and 32.8% aqueous solution of veroprograph (veropephrine 60%) in a ratio of 10:24 were mixed. The resulting vero- graph-fikol solution should have a density of 1.076-1.077. Dye-5% eosin solution in distilled water was mixed before use in a 4: 1 ratio with a 4.26% hypertonic sodium chloride solution.
The reaction can be performed by several methods, of which the most used was used - the "two-step method", proposed by the National Institute of Health in Bethesda (NIH method).
Isolation of lymphocytes: Venous blood volume of 5-6 ml was defibrinated in a container with a flat bottom containing glass beads d-6 mm by uniform shaking for 10-15 minutes; In a centrifuge tube were placed 2 ml of a verophagin-fikol solution; 4 ml of defibrinated blood, mixed in equal amounts with physiological saline, carefully layered onto a verophagin-fikol solution; Centrifuged for 20 minutes at 1500 rpm; Formed "cloud" of lymphocytes (a white ring between the density gradient and supernatant) was transferred to a clean test tube; 5 ml of Hanks solution was added to the lymphocyte suspension and centrifuged at 1500 rpm for 10 minutes for washing from a verophagin-fikol mixture; Laundering was repeated twice; Removing the supernatant, the concentration of lymphocyte suspension was adjusted to 2-4 × 10 3 in 1 μl with Hanks solution. Reaction of the reaction: a tube with a lymphocyte suspension was shaken; Using a Micron syringe of Hamilton, 1 μl of a suspension of lymphocytes was weighed into the wells of the Terasaki plate containing the histotyping sera (thawed immediately before the reaction); Incubated at room temperature for 45 minutes; 4-5 μl of standard rabbit complement was added to each well; Incubated in a dark place at room temperature for 1 hour; The plates were vigorously shaken to remove the vaseline oil; 1 μl of a 5% solution of eosin was added to each well; After 6-7 minutes, the plates were shaken to remove excess dye; The results were evaluated using a Biolam microscope at an increase of 10 × 20.
The reaction was evaluated by the ratio of toxic (colored) and non-toxic (shining) lymphocytes in the field of view of the microscope. If lymphocytes carrying a specific HLA-antigen are "recognized" by serum, then a cell lysis reaction occurs, which is determined by the penetration of the dye into the cells. The intensity of the reaction was evaluated according to the following criteria: 100% -90% cell death - dramatically positive (++++); 75% -50% cell death - positive (+++); 50% -25% cell death - poorly positive (++); 25% -10% cell death is a result of doubtful (- +). The results of the interaction of lymphocytes with the non-toxic serum of persons of the IV group of blood, which do not contain anti-HLA antibodies, were negative results of the reaction with antilymphocyte serum.
When evaluating the results, the principle of "battery life" was taken into account. The use of several immune sera of different series increases the accuracy of the results obtained and reduces the probability of error.
As a result, the patient had an antigen profile HLA-A3A9B12B35. The presence of HLA-B35 antigen allowed the patient to predict hemoblastosis and chronic hepatitis B. In the future, on the basis of bone marrow research, blood tests and cytochemical reactions, the patient was diagnosed with acute lymphoblastic leukemia. He was prescribed treatment according to the protocol of Heltser (1994). A complex study was conducted to identify signs of liver damage (study of markers of the hepatitis B virus by ELISA in the blood serum, biochemical blood test, ultrasound). Signs of liver damage could not be detected. During the course of polychemotherapy, the patient received more than 30 blood transfusions. When the blood serum was re-examined for 6 months, the patient was diagnosed with HBsAg, HBcAb, ABeAb, an increase in the transaminase level by a factor of 2, the level of bilirubin was within normal limits, and ultrasound of the liver determined a diffuse compaction of the liver parenchyma. When questioning the patient presented the previous complaints. The listed clinical and laboratory syndromes served as the basis for the diagnosis of chronic hepatitis B.
Thus, the immunogenetic study allowed predicting hemoblastosis and chronic hepatitis B in the presence of HLA-B35 antigen.
Example 2 . Patient B. of the history of the disease 854. Entered the department on 20.01.97. It has been observed since 1988 with the diagnosis of chronic lymphocytic leukemia. Specific treatment is given 2-3 times a year. Over the entire period of treatment, he received more than 50 blood transfusions. When the patient was included in the examination group, he complained of weakness, pain in the bones, joints, periodically heaviness in the right hypochondrium, epigastrium. From anamnesis: a year ago he suffered acute viral hepatitis B (with a characteristic clinic, jaundice, an increase in the level of transaminases 5-fold, bilirubin levels increased 2-fold, HBsAg was recorded in the blood serum). In the immunogenetic study (description of the procedure - example 1), the patient had an antigen profile HLA-A1A9B15B40, which allowed predicting the probability of chronic hepatitis B in the patient.
At inspection : an increase in the level of transaminases by 1.5 times, the level of bilirubin is not changed. Markers of the hepatitis B virus were determined by the ELISA method: HBsAg, HBcAb, HBeA. Liver ultrasound: the parenchyma of the liver is diffusely sealed. Follow-up of the patient for the next 6 months, with repeated monitoring of hepatitis B markers, made it possible to establish a diagnosis of chronic viral hepatitis B. Thus, the immunogenetic study allowed predicting the chronicity of hepatitis B in the presence of HLA-B40 antigen.
Example 3 . Patient T. of the history of the disease 5676. Enrolled in the department on 18.04.97. It is observed in the hematological department for 2 years with the diagnosis of multiple myeloma. Over the period of treatment, he received more than 50 blood transfusions and 6 courses of polychemotherapy. A complex study was conducted to identify signs of liver damage (study of markers of the hepatitis B virus by ELISA in the blood serum, biochemical blood test, ultrasound). Signs of liver damage could not be detected. In the immunogenetic study (description of the procedure - example 1), the patient has an antigen profile HLA-A2A19B12B27 (there is a haplotype A2 / B27), which allowed predicting the patient's resistance to infection with the hepatitis B virus. When monitoring a patient for 1 year with repeated control of markers Hepatitis B virus, the fact of infection with the hepatitis B virus was not detected. Thus, the immunogenetic study helped predict the resistance to infection with the patient's hepatitis B virus in the presence of a variety of risk factors (the state of immunosuppression, a large number of blood transfusions, massive drug load).
The proposed method has been used to improve the accuracy of predicting the features of the course of chronic viral hepatitis B in patients with hemoblastosis.
Advantages of the method is its high reliability, simplicity of laboratory performance, which indicates the possibility of wide application in the gastroenterological, hematological hospitals, oncological clinics.
CLAIM
A method for predicting the features of chronic hepatitis B in patients with hemoblastosis by immunogenetic investigation, characterized in that venous blood is detected by the antigens of the loci A, B, C of the HLA system and, in the presence of HLA-B35 antigen, predict chronic hepatitis B and hemoblastosis, in the presence of HLA- B40 predict the chronicity of hepatitis B, in the presence of haplotype A2 / B27 predict resistance to infection with the hepatitis B virus.
print version
Date of publication 30.03.2007gg




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.