| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2205661
![]()
ALLERGOTROPIN polynosis FOR TREATMENT AND METHOD OF TREATMENT polynosis
Name of the inventor: Petrov RV .; KHaitov RM .; Nekrasov AV .; Fedoseeva VN .; Puchkova NG .; Kamysheva VA .; Martynov AI
The name of the patentee: Petrov Rem; KHaitov Rahim Musaevich; Arkady V. Nekrasov; Fedoseeva Vera; Puchkova Natalia G.; Kamysheva Valeriya Alekseevna; Alexander I. Martynov; GP Institute of Immunology
Address for correspondence:. 115478, Moscow, Kashirskoye sh, 24, building 2, Institute of Immunology, Deputy. Director A.I.Martynovu
Starting date of the patent: 2001.07.13
The invention relates to medicine, namely immunology and allergy, and refers to the treatment polynosis. The essence of the invention is allergotropin containing allergoid pollen allergenic plants - timothy, birch, sage, obtained by the impact on the total fraction consisting of the major and minor allergens by MV 3-90 kDa - compounds containing reactive groups of the protein, modified mainly formaldehyde, and associated polyoxidonium. For the treatment administered to the patient polynosis allergotropiny, starting with dilution 10 -4 in the course of 15 injections. The technical result is based on obtaining allergotropinov allergoidnyh forms pollen allergens having compared to the native allergen and more pronounced immunogenic allergen properties.
DESCRIPTION OF THE INVENTION
The invention relates to medicine, namely to allergy and immunology, and relates to the treatment of allergic diseases such as hay fever.
Known formulation comprising a polymeric carrier polioksidony. For example, complex proteins gemmaglyutinina virus neuraminidase and associated with the carrier-polioksidoniem that increases immunogenicity (1).
Known drug for the treatment of hay fever, which is a culture supernatant of autologous mononuclear cells stimulated with phytohemagglutinin at a protein concentration of 150-200 ug / ml (2).
Known method of treating pollinosis and by electrophoresis endonasal drug substance, which is a culture supernatant of autologous mononuclear cells stimulated with phytohemagglutinin in an amount of 1.2 ml at a protein concentration of 150-200 ug / ml (3).
Currently, however, for specific immunotherapy (SIT) of persons who are allergic to pollen, used water-salt extracts, lack of which is the high content of ballast substances that are potential allergens, which creates a risk of the treatment - getting anaphylactic shock reactions.
The object of the present invention is to provide a highly efficient means and method for treating pollinosis using complex preparation (allergotropina) based allergoidnyh forms pollen allergens and immunomodulator - polioksidonija.
Allergotropin allergoidnyh based on pollen allergens forms polioksidonija and has, in comparison to the native allergen, more pronounced and immunogenic properties allergen (levels IgG-blocking antibodies in laboratory animals hyposensitization 3 times higher than for the native allergen desensitization).
To solve this problem and to achieve the specified technical result is proposed group of inventions united by a common inventive concept.
The drug for the treatment of allergotropin polynosis allergoid pollen contains conjugated to a synthetic immunomodulator-polioksidoniem while allergoid get exposure on the total fraction consisting of the major and minor allergens by MV = 3-90 kDa - compounds containing reactive groups of the protein, modified preferably formaldehyde.
Allergoid can be obtained by exposing the total fraction consisting of the major and minor allergens (with MW = 3-90 kDa), aldehydes or other compounds that block the protein-reactive group, in this case - with formaldehyde. Allergoid is a chemically modified pollen allergen and is superior to the latter, since the chemical modification of a decrease in the risk of allergenicity and guidance of anaphylactic shock.
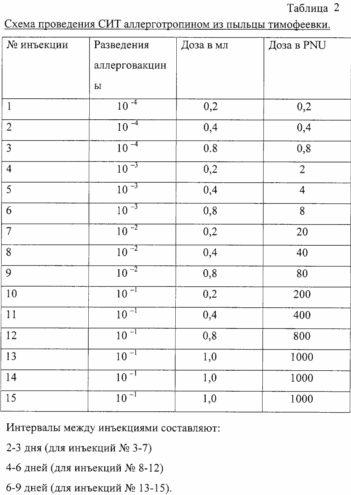
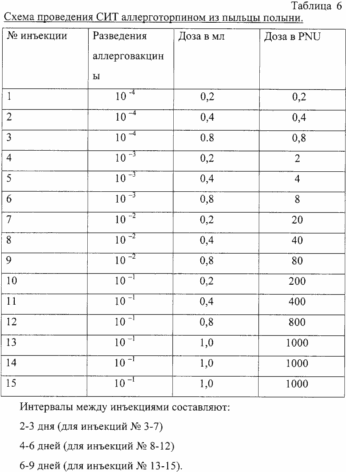
A method of treatment and those who are allergic to pollen: timothy, birch, sage, using allergotropina. Allergotropin drug is administered to the patient with the dilution 10 -4 in the course of treatment up to 15 injections.
Among the therapeutic and preventive measures aimed at combating atopic allergic diseases, the most important step is the specific hyposensitization therapy, which provides Etiopatogenetichesky approach in the treatment of patients suffering from pollen allergoid. However, the use of existing commercial allergens does not satisfy all the requirements of clinicians, allergists, since the drugs have pronounced allergenic activity and in some cases encourage the emergence of local and systemic allergic reactions, especially in highly sensitive patients. These complications usually lead to the fact that the rate of pre-specific immunotherapy (SIT) becomes longer. There are barriers to achieving optimal dose prior to the final flowering season of allergenic plants, which significantly affects the effectiveness of the treatment in patients who are sensitive to pollen.
To eliminate these drawbacks aqueous pollen extracts salt chemically modified formaldehyde (4, 5), glutaraldehyde (6, 7), urea (8), polyethylene glycol (9, 10).
Processing allergen formaldehyde promotes adherence of amino acids comprising the allergen to each other. This process of condensation of the protein molecules with low molecular weight compounds that contribute to the increase in molecular weight was studied Raikis BI Voronin and NI 1987 (11). The increase in molecular weight causes allergoid adjuvant their effect on the body. The presence in the incubation medium with formaldehyde lysine and arginine, in some cases enhances immunogenicity allergoids.
Some authors believe that should be used in medicinal preparations as much as possible the complex components of pollen with allergenic activity, can cause sensitization of patients. It should be noted that regardless of the degree of purification of crude allergen can achieve drastic reduction of allergenicity and immunogenicity of conservation allergoidnyh forms allergen.
It is possible that some protein compounds, being more labile than the major allergens are inactivated during formalinizatsii.
Gomotsitotropnye antibodies are sensitive to the action of 2-mercaptoethanol, they observed a significant decrease.
Gomotsitotropnye allergoid antibody to rabbits appear at a later time, in comparison with the allergen.
Differences allergoid fractional composition and source of the allergen, as defined in gel filtration and electrophoresis explained, seem to influence the dialyzed extract formaldehyde and condensation process development, "crosslinking" high and low molecular weight components of the original allergen methylene bridges.
There is reason to believe that the low molecular weight components of the original allergen are no ballast necessary to ensure their safety in allergoid.
At the heart of manufacture allergoid on the interaction of reactive aldehyde groups with amino groups of the allergen. Formalinized allergen contains a mixture of components with different molecular weights, mainly dimers and trimers, which is due to the accession to the allergen molecule relatively low-molecular nitrogen compounds, and due to the polymerization process.
Turning allergen allergoid by chemical modification in the presence of formaldehyde, which leads to the interaction of the high and low molecular weight components, it helps to preserve the properties allergoid induce the synthesis of antibodies. This effect is enhanced by rigid and stable structure allergoid molecule resistant to the effects of denaturants unlike the original allergen, but also contributes to a prolongation of action allergoid because its destruction in the body of the patient will be slower.
N.A.Illyutovich (1983) observed that the content of the specific IgG-antibodies in patients treated allergoid, 1.7 times the level of 'blocking' antibodies in patients treated with allergen (12).
A direct relationship between the values of the final dose and the content of "blocking" antibodies in patients treated with allergen.
Reduction of allergenicity of allergens in polymerized connected, on the one hand, with a decrease in the number of antigenic determinants by their reaction with aldehyde groups, on the other hand - for inaccessibility reagin other determinants hidden within the high molecular weight polymer. Positive impact on the immunogenicity has a molecular weight increase and the creation of more stringent intramolecular bonds.
Production of therapeutic allergen of high purity is costly and time-consuming process. This is compounded by the fact that in allergic diseases most frequently observed polisensibilizatsiya to many allergens diverse nature, and cleaning each of them has its own characteristics.
One of the indisputable advantages allergoidnyh allergies when properly designed scheme SIT is that he has not caused systemic reactions such as generalized urticaria, itching, dryness in the throat, nasal symptoms with asthma and without it. During the SIT allergen eliminated emerging systemic reactions with antihistamines.
Allergoid resulting from the processing of allergen glutaraldehyde has, in our view, a number of disadvantages: the preparation is not stable for 6 months continued polymerization and depolymerization. Unknown path glutaraldehyde utilization in the human body. The polymerization reaction is not controlled, it can not be directed synthesis.
One of the stages of development of drugs for the treatment of pollen allergy (allergy to pollen of timothy grass, birch and other. Plants) was the creation of allergotropinov based allergoids pollen of these plants, associated with high molecular weight immunomodulator polioksidoniem, ie drugs with strong immunogenic properties, but have a low allergenic activity. In the development of these drugs have been taken into account the advantages of its constituent components, each of which is used in medical practice: allergoid and polyoxidonium. Polioksidony - domestic drug with broad spectrum of pharmacological action, which is unparalleled in the world both in structure and properties [1]. The drug is used as an immunomodulator, detoksikant, immunostimulatory and sustained release carrier of pharmacologically active compounds. Polioksidony (ON) is approved for use in 1996, reg.nomer 96/302/9, FS 42-3906-00. The three-year experience in the application software has shown its high clinical efficacy in treatment of almost all secondary immunodeficiency states. Software belongs to a class of water-soluble derivatives of hetero-aliphatic polyamines. Software has a broad spectrum of immunopharmacological properties, fundamentally important to create on its basis of vaccines, high bioavailability, ability of excretion, use safety. Software in a wide range of doses activates macrophage link humoral immune response, possesses anti-infective and anti-tumor activity of cell membranes increases the resistance to the cytotoxic effect and reduces the toxicity of drugs when co-administered.
When creating vaccinated and medicinal products is a new generation of high molecular immunostimulant carrier antigens and allergoids software provides greater stability to antigens, increased immunogenicity at lower vaccination dose of antigens, more efficient formation of immunological memory to antigens and increase preventive effectiveness of the vaccine, but also increased safety of the vaccine. Modification of various nature macromolecular antigen immunostimulant software allows you to enhance the immunogenic activity. Accession to allergoid software reduces the allergenic activity, improve the safety of the drug and reduce the risk of complications when administered to sensitized body. Binding allergoids with software makes it possible to significantly enhance the immune response, stimulate the production of high levels of allergen-specific IgG-antibodies that protect the patient in contact with hay fever pollen allergensoderzhaschey in his body.
When developing a new generation of allergotropinov polyoxidonium choice as a high-immunostimulatory carrier antigen was driven by the positive experience obtained earlier.
The most promising areas in the further development of modified forms of allergens, we believe allergotropiny: conjugated forms allergoid and immunostimulant.
At present, it developed and produced allergotropin created on the basis of allergoids timothy pollen, birch and sage and a polyionic media - synthetic high molecular immunostimulant - polyoxidonium.
example 1
Pollen from timothy Allergotropin called "Timpol" represents allergoid timothy pollen prepared from a purified allergen, a freeze-dried extract of pollen associated with synthetic macromolecular polioksidoniem immunomodulator (copolymer of N-oxide and 1,4-etilenpiperazina (N-carboxyethyl) 1, 4 etilenpiperaziny bromide). This bluish powder containing conjugate allergoidnoy major and minor forms of pollen allergens (M.W with from 3 to 90 kDa) and polioksidonija.
Allergotropin liquid (for injection), prepared by dissolving the dry formulation in 1 ml of a dilution of standard fluid for allergen, it contains 1,000 ± 200 PNU timothy pollen allergoids.
Allergotropin stored in a dry, dark place at a temperature of 4 to 8 o C. Shelf life 4 years. Allergotropin tested on a limited number of volunteers.
To prepare the diluent solution to 1 ml of 0.9% solution 0.5 ml of sodium chloride Tween-20, then 0.1 M Na 2 (HPO) 4 solution to pH 7.3 ± 0.1.
The preparation contains a specific active form allergoidnuyu timothy pollen allergen and has the ability to interact with specific thereto IgG-antibody positive control serum of patients with hypersensitivity to pollen of timothy that detect peroxidase conjugate anti-monoclonal antibodies IgS forming color staining with the substrate mixture. Determination is carried out ELISA.
In the reaction using the comparison reference serum: positive control and negative control sera. The control positive serum contains antibodies specific to IgG-timothy pollen allergen, the level of which corresponds to the optical density of not less than 0.8. Negative control serum contains allergen specific IgG-antibodies.
To assess allergotropina immunogenic activity in animal experiments, mice were given twice at weekly intervals intraperitoneally administered 100 micrograms of the drug. After 3, 5, 6, 7 weeks after the start of immunization, the animals are sacrificed and their sera determined by ELISA of circulating IgG-antibodies.
Immunogenicity allergotropina studied in patients with hypersensitivity volunteers timothy pollen, treated allergotropinom. Allergotropin at a dose of 0.1 mg of protein and 10 mg allergoid polyoxidonium stimulates the growth of allergen-IgG-antibody titers to timothy pollen in sera of patients after administration of the drug.
Allergotropin from timothy pollen used for immunotherapy of patients with hay fever are allergic to the pollen of timothy.
Allergotropin prescribed for specific immunotherapy if indicated: hay fever with hypersensitivity to pollens of timothy.
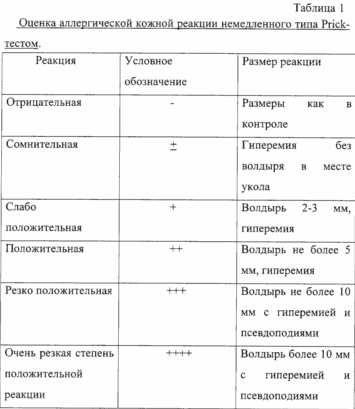
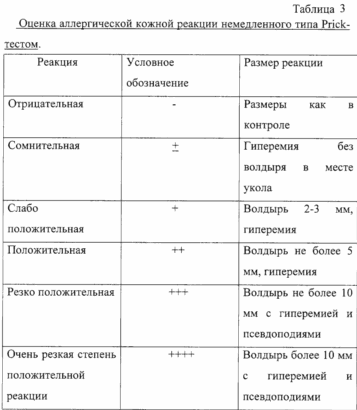
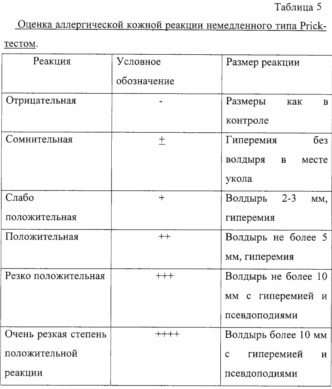
Prior to administration of therapeutic doses allergotropina carried out assessment of individual patient's sensitivity to the drug. The assessment carried out by prick-titration. Along with skin prick test formulation with allergotorpinom allergen and all subjects were conducted skin prick tests with a 0.01% solution of histamine dihydrochloride (positive control) and a standard test for fluid control of pollen allergens (negative control).
In formulating skin prick test reactions account for over 20 minutes, 6 - 24 hours (immediate type reactions).
The initial concentration of the drug is one that gives slightly positive or dubious reactions in patients with CIDP-titration allergovaktsiny.
The intervals between injections can be reduced and increase depending on patient tolerability. After completion of the introduction of possible SIT supporting doses of 1.0 ml (conc. 1000 PNU / ml) at intervals of 10-12 days. Introduction maintenance doses allergotropina and condition of the patient record in the history of the disease (medical card) and blog SIT.
When administered therapeutic doses may experience allergotropina local allergic reactions of immediate and delayed type. In these cases it is recommended to further administration per os to the patient an antihistamine and the dose administered and allergotropina divided into two (two-handed).
example 2
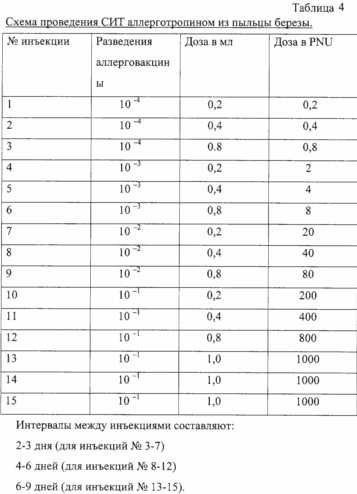
Allergotropin birch pollen called "Berpol" represents allergoid birch pollen allergen prepared from purified, freeze-dried extract of pollen associated with synthetic macromolecular polioksidoniem immunomodulator (copolymer of N--1,4 etilenpiperazina oxide and (N-carboxyethyl) -1 4-etilenpiperaziny bromide). This is slightly yellowish powder containing conjugate allergoidnoy form major and minor birch pollen allergens by MV = 3-90 kDa and polyoxidonium.
Allergotropin liquid (for injection), obtained by dissolving the dry formulation in 1 ml of a dilution of standard fluid for allergen, it contains 1,000 ± 200 PNU birch pollen allergoids.
Allergotropin stored in a dry, dark place at a temperature of 4 to 8 o C. Shelf life 4 years. Allergotropin tested on a limited number of volunteers.
To prepare the diluent solution to 1 ml of 0.9% solution 0.5 ml of sodium chloride Tween-20, then 0.1 M Na 2 (HPO) 4 solution to pH 7.3 ± 0.1.
The preparation contains a specific active form allergoidnuyu birch pollen allergens (c M.W = 3-90 kD) and has the ability to interact with specific reference thereto blood serum IgG-positive patients with antibodies to the birch pollen hypersensitive that detect peroxidase conjugate monoclonal anti- IgG-antibody staining color forming substrate to the mixture. Determination is carried out ELISA.
In the reaction using the comparison reference serum: positive control and negative control sera. The control positive serum contains IgG-antibodies specific to birch pollen allergen, the level of which corresponds to the optical density of not less than 0.8. Negative control serum contains allergen specific IgG-antibodies.
To assess allergotropina immunogenic activity in animal experiments, mice were given twice at weekly intervals intraperitoneally administered 100 ug allergotropina. After 3, 5, 6, 7 weeks after the start of immunization, the animals are sacrificed and their sera determined by ELISA of circulating IgG-antibodies.
Immunogenicity allergotropina studied in patients volunteers with hypersensitivity to birch pollen, treated allergotropinom. Allergotropin at a dose of 0.1 mg of protein and 10 mg allergoid polyoxidonium stimulates the growth of allergen titers of IgG-antibodies to birch pollen in sera of patients after administration of the drug.
Allergotropin of birch pollen is used for immunotherapy of patients with hay fever are allergic to birch pollen.
Allergotropiny prescribed for specific immunotherapy if indicated: hay fever with hypersensitivity to birch pollen.
Prior to administration of therapeutic doses allergotropina carried out assessment of individual patient's sensitivity to the drug. The assessment carried out by prick-titration. Along with skin prick test formulation with allergotropinom allergen and all subjects were conducted skin prick tests with a 0.01% solution of histamine dihydrochloride (positive control) and a standard test for fluid control of pollen allergens (negative control).
In formulating skin prick test response into account in 20 minutes, 6-24 hours (immediate type reactions).
The initial concentration of the drug is one that gives slightly positive or dubious reactions in patients with CIDP-titration allergotropina.
The intervals between injections can be reduced and increase depending on patient tolerability. After completion of the introduction of possible SIT supporting doses of 1.0 ml (conc. 1000 PNU / ml) at intervals of 10-12 days. Introduction maintenance doses allergotoropina and condition of the patient record in the history of the disease (medical card) and blog SIT.
When administered therapeutic doses may experience allergotropina local allergic reactions of immediate and delayed type. In these cases it is recommended to further allergotropina administration per os to the patient an antihistamine and the dose administered and allergotropina divided into two (two-handed).
example 3
Allergotropin pollen from Artemisia called "polpolya" is allergoid Artemisia pollen made from a purified allergen, a freeze-dried extract of pollen associated with synthetic macromolecular immunomodulator polioksidoniem (a copolymer of N-oxide and 1,4-etilenpiperazina (N-carboxyethyl) -1 4-etilenpiperaziny bromide). This is slightly greenish powder containing conjugated form major and minor allergens of pollen of Artemisia (MW = 3-90 kDa) and polyoxidonium.
Allergotropin liquid (for injection) was obtained by dissolving the dry formulation in 1 ml of a dilution of standard fluid for allergen, it contains 1,000 ± 200 PNU Artemisia pollen allergoids.
Allergotropiny stored in a dry, dark place at a temperature of 4 to 8 o C. Shelf life 4 years. Allergotropin tested on a limited number of volunteers.
To prepare the diluent solution to 1 ml of 0.9% solution 0.5 ml of sodium chloride Tween-20, then 0.1 M Na 2 (HPO) 4 solution to pH 7.3 ± 0.1.
The preparation contains a specific active form allergoidnuyu Artemisia pollen allergen and has the ability to interact with specific thereto IgG-antibody positive control serum of patients hypersensitive to Artemisia pollen that detect peroxidase conjugate Monoclonal Anti-IgG-antibody staining color forming substrate to the mixture. Determination is carried out ELISA.
In the reaction using the comparison reference serum: positive control and negative control sera. The control positive serum contains IgG-antibodies specific to Artemisia pollen allergen, the level of which corresponds to the optical density of not less than 0.8. Negative control serum contains allergen specific IgG-antibodies.
To assess allergotropina immunogenic activity in animal experiments, mice were given twice at weekly intervals intraperitoneally administered 100 ug allergotropina. After 3, 5, 6, 7 weeks after the start of immunization, the animals are sacrificed and their sera determined by ELISA of circulating IgG-antibodies.
Allergotropiny of Artemisia pollen is used for immunotherapy of patients with hay fever are allergic to the pollen of Artemisia.
Allergotropiny prescribed for specific immunotherapy if indicated: hay fever with hypersensitivity to pollen of Artemisia.
Prior to administration of therapeutic doses allergotropina carried out assessment of individual patient's sensitivity to the drug. The assessment carried out by prick-titration. Along with skin prick test formulation with allergotropinom allergen and all subjects were conducted skin prick tests with a 0.01% solution of histamine dihydrochloride (positive control) and a standard test for fluid control of pollen allergens (negative control).
In formulating skin prick test response into account in 20 minutes, 6-24 hours (immediate type reactions).
The initial concentration of the drug is one that gives slightly positive or dubious reactions in patients with CIDP-titration allergotropina.
The intervals between injections can be reduced and increase depending on patient tolerability. After completion of the introduction of possible SIT supporting doses of 1.0 ml (conc. 1000 PNU / ml) at intervals of 10-12 days. Introduction maintenance doses allergotropina and condition of the patient record in the history of the disease (medical card) and blog SIT.
When administered therapeutic doses may experience allergotropina local allergic reactions of immediate and delayed type. In these cases it is recommended to further allergotropina administration per os to the patient an antihistamine and the dose administered and allergotropina divided into two (two-handed).
The invention is illustrated in Table. 1-6.
 |
 |
 |
 |
 |
 |
USED BOOKS
1. Patent RU 1580617, 1997, IPC A 61 K 39/145.
2. Patent RU 2139742, 1999 A 61 K 35/14.
3. Patent RU 2139742, 1999 A 61 K 35/14.
4. Carter EB, Detoxified pollen extract., USA, 1935, Pat. 20198088.
5. Marsh DG, Lichtenstein LM, Studies on allergoid., J. Allergy, 1970, v.45, n2, p. l25.
6. Patterson R., Suszko IM, McIntire FC, Polimerised Ragweed antigen EI Preparation and immunologic studies., J. Immunol., 1973 a, v. 110, n.5, p. l402.
7. Patterson R., Suszko IM, Pruzansky JJ et al., Polimerised radweed antigen E. II. In vivo elimination studies and reactivity with antibody systems. J. Immunol., 1973 b, v. 110, n.5, p. 1413.
8. King T. P., Norman PS, Tao N., Chemical modification of the major allergen of ragweed pollen antigen E., Immunochemistry, 1974, v. 11, p. 83.
9. Lee WJ, Sehon AH, Suppression of reaginic antibodies with modified allergens. Reduction in allergencity of protein allergens by conjugation to polyethylene glycol. Int. Arch. Allerg. Appl. Immunol., 1978, v. 6, n. 2, p.159.
10. Lee WJ, Sehon AH, Suppression of reaginic antibodies with modifided allergtns. Abrogation of reaginic antibodies with allergens conjugated to polyethylen glycol. Int. Arch. Allergy appl. Immunol., 1978, v.56, n. 3, p. 193.
11. Raikis BN Voronkin NI "Therapeutic allergens." L., Med., 1987, 155 Art.
12. Illyutovich NA Biological properties allergoid of ragweed pollen. Kand. diss. 1983.
CLAIM
1. Allergotropin polynosis treatment, characterized in that it comprises the allergenic plant pollen allergoid obtained by exposing the total fraction consisting of the major and minor allergens M.W 3-90 kDa - compounds containing reactive groups of the protein, modified mainly formaldehyde, and an associated synthetic immunomodulator - polyoxidonium.
2. Allergotropin according to claim 1, characterized in that it contains allergoid timothy pollen, birch or wormwood.
3. A method of treating polynosis, characterized in that it is administered to a patient according to claim allergotropin. 1, the introduction of the drug starts from 10 -4 dilution at 15 treatment injections.
4. The method of claim. 1, characterized in that the treatment is carried polynosis caused by timothy grass pollen, birch or sage.
print version
Publication date 02.04.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.