| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Technology market / Current inventions and models / Back / |
|
INVENTION
Patent of the Russian Federation RU2285711
METHACRYLATE CONSTRUCTION ADHESIVE
![]()
Applicant's name:
The name of the inventor:
The name of the patent owner:
Address for correspondence: 129010, Moscow, ul. B. Spasskaya, 25, p. 3, LLC "Law firm Gorodissky & Partners", Pat. G.B. Yegorova, registration number 513
The effective date of the patent: 2003.04.22
The invention relates to methacrylate structural adhesives and to methods of using these adhesives. The technical task is to create an adhesive with an efficient and uniform distribution of the load over the joint, with high physical and mechanical properties at a high temperature, at the same time suitable for operation without significant surface preparation. The task is achieved by the fact that the adhesive comprises a monomeric component and a catalytic component. In a first embodiment of the invention, the monomer component comprises a first elastomeric material comprising a block copolymer of styrene and isoprene, a second elastomeric material comprising a block copolymer of styrene and butadiene. In a second embodiment, the monomer component comprises a first elastomeric material comprising a block copolymer of styrene and butadiene, and a crosslinked rubber comprising a copolymer of acrylonitrile and butadiene. In another embodiment, isophthalic acid is used as a polymerizable acidic monomer in combination with a phosphate ester. Alternatively, the monomer component includes a cohesive fracturing regime promoter, which is an esterified rosin or talc.
DESCRIPTION OF THE INVENTION
The present invention relates to methacrylate structural adhesives and to methods for using such adhesives.
Structural adhesives are well known for joining metal to metal, metal to plastic and plastic to plastic. Structural adhesives are an attractive alternative to mechanical bonding methods, such as riveting or spot welding, because structural adhesives distribute stress under load over large surfaces rather than concentrating such stresses at several points. When using structural adhesives, it is possible to reduce or eliminate the cost of finishing, since there is no need to hide fasteners to obtain an aesthetically appealing appearance. The use of structural adhesives makes it possible to obtain products more cleanly and quietly, because these glues are made without water, dust and noise. In addition, they can be used to connect a variety of materials without significant surface preparation.
Despite their attractiveness, the known structural adhesives have several potential drawbacks. Despite the fact that known structural adhesives have good characteristics at high temperature and good durability, the connection they create is tough. The rigid joint unevenly distributes the load over the joint, causing the load at the joint edges to be higher than the load in the middle of the connection. Thus, when the two pieces are joined together overlap, increasing the size of the overlap does not lead to a significant increase in the strength of the joint. Moreover, uneven loading on rigid structural adhesives can damage the part, since the glue will not break down, but rip off the paint and coating or in some cases tear the fiber of reinforced plastic.
In addition, structural adhesives are known which overcome the problem of stiffness. These elastic adhesives evenly distribute the load over the joint. The result of this phenomenon is that the load is effectively absorbed and distributed. However, known flexible structural adhesives have unacceptably poor characteristics at high temperatures and insufficient durability.
Therefore, the inventors have realized the need for a structural adhesive that has excellent flexibility, good heat characteristics and good durability, and which is at the same time usable without substantial surface preparation. The inventors have realized the need for a structural adhesive that causes minor damage to painted or coated parts.
SUMMARY OF THE INVENTION
The present invention relates to several compositions for use as a structural adhesive. The compositions include a monomer component and a catalytic component. In one aspect, the monomer component comprises a first elastomeric material comprising a block copolymer of styrene and isoprene, a second elastomeric material comprising a block copolymer of styrene and butadiene, and a crosslinked rubber comprising a copolymer of acrylonitrile and butadiene. In a second aspect, the monomer component comprises a first elastomeric material comprising a block copolymer of styrene and butadiene, and a crosslinked rubber comprising a copolymer of acrylonitrile and butadiene. Isophthalic acid is used as a polymerizable acidic monomer in combination with a phosphate ester. The monomer component includes a cohesive failure mode promoter, which is an esterified rosin or talc.
DETAILED DESCRIPTION OF THE INVENTION
The described structural adhesive compositions include at least two components. The first or monomer component of the composition may have several subcomponents, including a methacrylate ester monomer, additional monomers and at least one elastomeric material. A monomer component, and may include, inter alia, adhesion promoters, crosslinked rubbers, initiators that are tertiary amines, inhibitors, film edge setting promoters at the edges, thixotropic agents, antioxidants, plasticizers, talc and cohesive fracture promoters. The second or catalytic component of the composition includes a polymerization catalyst.
Monomers that are methacrylate ester include monomers, where the alcohol moiety of the ester group contains from one to eight carbon atoms. Examples of such ester monomers are methyl methacrylate (MMA), ethyl methacrylate, 2-ethylhexyl methacrylate, cyclo-10-hexyl methacrylate, and mixtures thereof. The preferred ester monomer is MMA.
Additional monomers that can be used in combination with methacrylate ester monomers are acrylate esters, wherein the alcohol portion of the ester contains from one to eight carbon atoms, examples of which are methyl acrylate, ethyl acrylate, butyl acrylate and 2-ethylhexyl acrylate. Other useful monomers are acrylonitrile, methacrylonitrile, styrene, vinyltoluene and the like.
Other additional monomers that can be used in combination with methacrylate ester monomers are those capable of polymerization containing ethylenic unsaturation mono- or polycarboxylic acids. Examples of such acids are acrylic acid, methacrylic acid (MAK), isophthalic acid (IPA), crotonic acid, maleic acid and fumaric acid. Preferred acids are MAA or IFC.
The at least one elastomeric material can be selected from block copolymers of styrene and isoprene or butadiene, which contain from about 10 to about 50 weight percent styrene. For example, suitable block copolymers of styrene and isoprene include block copolymers with a styrene content of about 10-20 wt%, with copolymers having a styrene content of about 17 wt%. One such block copolymer is Kraton TM D-1117 from Shell Oil. Suitable block copolymers of styrene and butadiene include block copolymers with a styrene content of about 10-50 wt%. One preferred copolymer of butadiene and styrene has a styrene content of about 18% by weight, while the other has a styrene content of about 44-45% by weight. Examples of preferred block copolymers include Kraton TM D-KX-222C from Shell Oil and Stereon ™ 840 and 857A from Firestone Polymers. Additional useful elastomeric materials include polychloroprene and nitrile rubber (copolymers of acrylonitrile and butadiene).
The adhesion promoter increases the ability of the composition to adhere to galvanized steel. Although known adhesion promoters can be used, phosphate ester is preferred, since elasticity is not sacrificed to increase adhesion. The phosphate ester is preferably selected so that it corresponds to the monomer that is a methacrylate ester. One preferred adhesion promoter is a monoethacrylate phosphate ester, commercially available under the trade name CD 9050 from Sartomer of Exton, PA.
The crosslinked rubber increases the impact resistance and flexibility of the composition and is preferably chosen so that it readily dissolves in other subcomponents of the monomer component. Preferably, the crosslinked rubber has an average particle size of less than 0.5 mm. One suitable cross-linked rubber is nitrile rubber (copolymers of acrylonitrile and butadiene). For example, suitable nitrile rubbers include rubbers with an acrylonitrile content of about 30-35 wt%, with rubbers having an acrylonitrile content of about 33 wt%. One example of nitrile rubber is Zealloy TM 1422 from Zeon Chemicals. Another suitable crosslinked rubber may be an acrylate terpolymer such as the Sunigum ™ line polymers produced by Goodyear Chemical.
The initiator, which is a tertiary amine, helps accelerate the reaction of monomers that are a methacrylate ester with a polymerization catalyst and is selected from N, N-dimethylaniline, N, N-dimethyltoluidine (DMT), N, N-diethylaniline, N, N-diethyltoluidine , N, N-bis [dihydroxyethyl] -p-toluidine, N, N-bis [dihydroxypropyl] -p-toluidine and the like.
The inhibitor prolongs the shelf life of the composition. Known inhibitors may be used, but benzoquinones are preferred, with naphthoquinone being most preferred. The film setting time promoter along the edges, as the name suggests, increases the length of time during which the film does not form on the adhesive. Suitable film setting time promoters at the edges include various waxes, and solid paraffin, such as the International Group IGI 1977, is preferred. The thixotropic agent can be used to increase the viscosity of the monomer component, and thus to prevent run-off or build-up of glue deposits when applied vertically. Suitable thixotropic agents are known to those skilled in the art and include polyamide thixotropic agents, with King Industries Disparlon 6100 being preferred. Suitable antioxidants are known to those skilled in the art, with the preferred antioxidant being 2,6-di-tert-butyl-p-cresol, and known as ionol. To reduce the viscosity of the monomer component, a plasticizer can be used. Known plasticizers can be used, with the preferred plasticizer being diisodecyl adipate (DIDA).
A monomer component and may include a cohesive fracture mode promoter. When the structural adhesive is destroyed, it is desirable that the adhesive remain on both of the resulting parts. This is called the cohesive failure mode. Examples of cohesion fracture promoters include any esterified rosin, such as tall oil, gum rosin, wherein the extraction rosin is the preferred type of esterified rosin. As a promoter of the cohesive failure regime, talc may be used. Suitable talc is selected based on the cost. A variety of esterified rosins and / or talcs can be used as a promoter of the cohesive failure mode in combination or separately.
The monomer component may be prepared by the following procedure or procedures known to those skilled in the art. Preferably, a solution of each elastomeric material is prepared in the methacrylate ester monomer. Similarly, if a cohesive fracture mode promoter, antioxidant or inhibitor is to be used, solutions are prepared in the methacrylate ester monomer. In addition, a solution of the film setting time promoter is prepared along the edges in xylene. Other components can be added undiluted.
Although you can use any order of adding subcomponents to a monomeric component, the following order is preferred. To the solution (s) of the elastomer (s), a solution of the cohesive failure mode promoter is added, if present. Then, the remainder of the undiluted methacrylate ester monomer is added, followed by a plasticizer, an adhesion promoter, a film boundary setting promoter at the edges, an antioxidant, an inhibitor, additional monomers and a tertiary amine initiator. It is not necessary to include all the subcomponents in each monomeric component. The included subcomponents are mixed. Then talcum and crosslinked rubber are added, while slowly increasing the agitation speed. Then a thixotropic agent is added and stirring is continued. The mixer is stopped and the mixture is allowed to stand. To ensure that the thixotropic agent is properly activated and ensure the complete swelling of the crosslinked rubber, it is possible to re-mix the mixture and allow it to stand. After it is allowed to stand, the mixture is stirred to obtain a uniform consistency. Finally, the mixture is stirred under vacuum to remove trapped air. Typically, the amount of monomer that is a methacrylate ester can be increased to compensate for the losses associated with the application of the vacuum.
In one aspect, the monomer component that has an improved shear strength when overlapping at a high temperature contains the subcomponents listed in Table 1 :
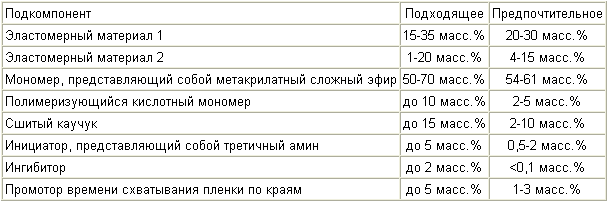
In another aspect, a monomer component that has improved adhesion to galvanized steel and aluminum while retaining good flexibility contains the sub-components listed in Table 2 :
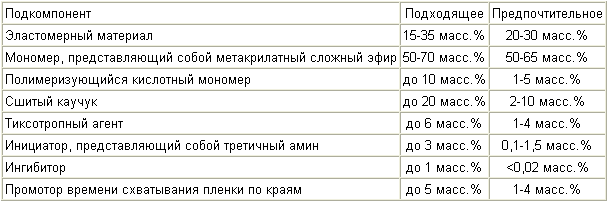
In a third aspect, the monomer component that has an improved resilient reduction, and at the same time has a generally cohesive failure mode, contains the subcomponents listed in Table 3 :
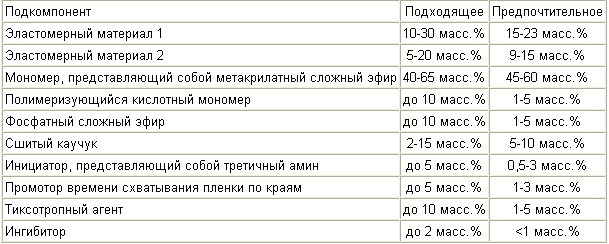
In a fourth aspect, the monomer component that has an improved resilient recovery comprises the subcomponents listed in Table 4 :
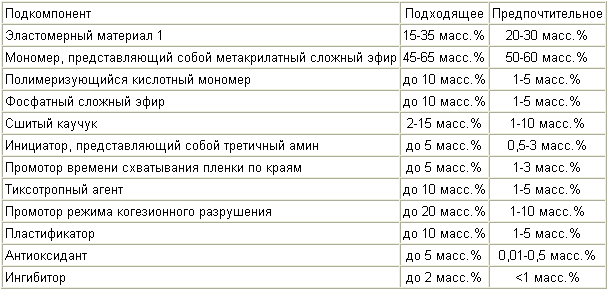
In another aspect, the monomeric component comprises the subcomponents listed in Table 5 :

The catalytic component of the composition is a polymerization catalyst. Suitable catalysts include free-radical generators that initiate the polymerization of the monomer component. Such catalysts are peroxides, hydroxyperoxides, peresters and peracids. Examples of these catalysts are benzoyl peroxide, cumene hydroperoxide, tert-butyl hydroperoxide, dicumyl peroxide, tert-butylperoxyacetate, tert-butyl perbenzoate, di (t-butyl) azodiisobutyronitrile and the like. As a catalyst, radiant energy, for example ultraviolet light and heat, can be used. A preferred catalyst is a paste of 18 wt% anhydrous benzoyl peroxide.
When used, the two components of the composition of the invention are combined and then applied to the parts to be joined. Alternatively, the two components can be applied individually to the parts that need to be connected, and connecting the parts serves to connect the two components. When radiant energy is used as the second component, the radiant energy can be influenced by the first component before or after the first component is applied to the part.
Typically, the ratio of the monomer component to the catalytic component of the composition may be in the range of from 30: 1 to about 1: 1. More preferably, the ratio is from 15: 1 to 5: 1. Most preferably, the ratio of the monomer component to the catalytic component is 10: 1.
EXAMPLES
Example 1
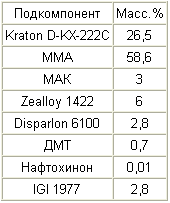 |
Prepare and experience the following compositions. The test samples of the monomeric component are prepared as described above, where the weight% refers to the composition of the monomer component. For all test samples, the catalyst component is an 18% paste of benzoyl peroxide. To compare the test samples, a ratio of 10: 1 (monomer component: catalytic component) is used. To use the structural adhesive, the monomer component is mixed with the catalyst component and applied to the parts, which are then joined together. The tensile strength, elongation and modulus of the resulting compositions are measured by the procedures set forth in ASTM D638-95, while the shear strength of the lapped joint is measured according to ASTM D1002-94. Elastic recovery of the composition is calculated by obtaining a strength curve from the load, starting from the composition module. The linear part of the curve corresponds to the elastic restoration of the composition. |
A 35% Kraton D-1117 solution is prepared in MMA, and as a 35% solution of Stereon 840 in MMA and a 1% solution of naphthoquinone in MMA. In addition, a 10% solution of IGI 1977 in xylene is prepared.
To a solution of Kraton D-1117, a solution of Stereon 840 is added, followed by addition of the remaining amount of MMA. Then, in order to add a solution of IGI 1977, a solution of naphthoquinone, MAK and DMT. These subcomponents are mixed at about 800 rpm for 10 minutes. Zealloy 1422 is then added while slowly increasing the stirring speed to about 900 rpm and this rate is maintained for about 15 minutes. The mixture is allowed to stand for at least three hours, after which the mixture is stirred at about 1200 rpm for 20 minutes, obtaining a uniform consistency. The mixture is then stirred at about 50 rpm, while evacuating the mixture to remove trapped air.
Test results for example 1

Duraplate TM , sold by Wabash National, is a plastic placed in the middle of two layers of steel. Duraplate TM and similar materials are commonly used in the manufacture of vehicles.
Example 2
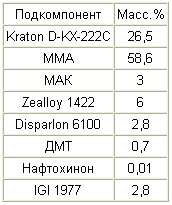 |
Prepare a 1% solution of naphthoquinone in MMA, and prepare a 10% solution of IGI 1977 in xylene. A solution of Kraton D-KX-222C in MMA is prepared, to which IGI solution 1977, a solution of naphthoquinone, MAK and DMT, is added in order. These subcomponents are mixed at about 800 rpm for 10 minutes. Zealloy 1422 is then added while slowly increasing the stirring speed to about 900 rpm and this rate is maintained for about 15 minutes. Disparlon 6100 is then added and mixed for an additional 15 minutes at 900 rpm. The mixture is allowed to stand for at least three hours, after which the mixture is stirred at about 1200 rpm for 20 minutes, obtaining a uniform consistency. The mixture is then stirred at about 50 rpm, while evacuating the mixture to remove trapped air. |
Test results for example 2

Example 3
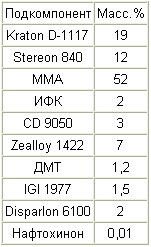 |
A 35% solution of Kraton D-1117 in MMA is prepared, and a 35% solution of Stereon 840 in MMA and a 1% solution of naphthoquinone in MMA are prepared. In addition, a 10% solution of IGI 1977 in xylene is prepared. To a solution of Kraton D-1117, a solution of Stereon 840 is added, followed by the addition of the remaining MMA. Then CD 9050, IGI solution 1977, naphthoquinone solution, IFC and DMT are added in order. These subcomponents are mixed at about 800 rpm for 10 minutes. Zealloy 1422 is then added while slowly increasing the stirring speed to about 900 rpm and this rate is maintained for about 15 minutes. Disparlon 6100 is then added and mixed for an additional 15 minutes at 900 rpm. The mixture is allowed to stand for at least three hours, after which the mixture is stirred at about 1200 rpm for 20 minutes, obtaining a uniform consistency. The mixture is then stirred at about 50 rpm, while evacuating the mixture to remove trapped air. |
Test results for example 3

Example 4
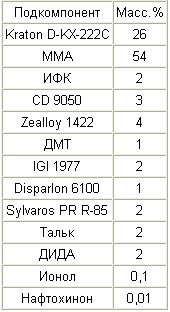 |
A 35% solution of Kraton D-KX-222C in MMA is prepared, and a 40% solution of Sylvaros PR R-85 in MMA, a 40% solution of ionol in MMA and a 1% solution of naphthoquinone in MMA. In addition, a 10% solution of IGI 1977 in xylene is prepared. A solution of Sylvaros PR R-85 is added to the Kraton D-KX-222C solution, after which the remaining amount of MMA is added. Then DIDA, CD 9050, IGI solution 1977, ionol solution, naphthoquinone solution, IFC and DMT are added in order. These subcomponents are mixed at about 800 rpm for 10 minutes. Then talcum and Zealloy 1422 are added, while slowly increasing the stirring speed to about 900 rpm, and this speed is maintained for about 15 minutes. Disparlon 6100 is then added and mixed for an additional 15 minutes at 900 rpm. The mixture is allowed to stand for at least three hours, after which the mixture is stirred at about 1200 rpm for 20 minutes, obtaining a uniform consistency. The mixture is then stirred at about 50 rpm, while evacuating the mixture to remove trapped air. |
Test results for Example 4

Example 5
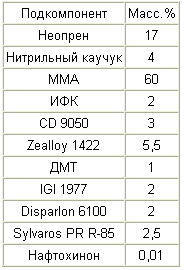 |
A 35% solution of neoprene in MMA is prepared, and a 35% solution of nitrile rubber in MMA, 40% solution of Sylvaros PR R-85 in MMA and 1% solution of naphthoquinone in MMA. In addition, a 10% solution of IGI 1977 in xylene is prepared. A solution of nitrile rubber and a solution of Sylvaros PR R-85 are added to the neoprene solution, after which the remaining amount of MMA is added. Then CD 9050, IGI solution 1977, naphthoquinone solution, IFC and DMT are added in order. These subcomponents are mixed at about 800 rpm for 10 minutes. Zealloy 1422 is then added while slowly increasing the stirring speed to about 900 rpm and this rate is maintained for about 15 minutes. Disparlon 6100 is then added and mixed for an additional 15 minutes at 900 rpm. The mixture is allowed to stand for at least three hours, after which the mixture is stirred at about 1200 rpm for 20 minutes, obtaining a uniform consistency. The mixture is then stirred at about 50 rpm, while evacuating the mixture to remove trapped air. |
Test results for Example 5

While the invention has been specifically described in connection with certain specific embodiments in practice and examples thereof, it is to be understood that this is given by way of illustration and not limitation and the scope of the appended claims is to be interpreted as broadly as the previous level allows Techniques.
CLAIM
A composition used as a structural adhesive comprising a subcomponent comprising 15-35% by weight of a first elastomeric material which is a block copolymer of styrene and isoprene, 1-20% by weight of a second elastomeric material which is a styrene block copolymer and Butadiene, 50-70% by weight of the methacrylate ester monomer, up to 10% by weight of the polymerizable acid monomer, up to 15% by weight of the crosslinked rubber, which is a copolymer of acrylonitrile and butadiene, up to 5% by weight of the initiator being A tertiary amine, up to 2% by weight of the inhibitor and up to 5% by weight of the film setting time promoter at the edges and a catalyst component.
The composition of claim 1, wherein the styrene-isoprene block copolymer has a styrene content of about 17%, the block copolymer of styrene and butadiene has a styrene content of about 18%, and the acrylonitrile-butadiene copolymer has an acrylonitrile content of about 33%.
A composition used as a structural adhesive comprising a subcomponent comprising 15-35% by weight of a first elastomeric material, 50-70% by weight of a methacrylate ester monomer, up to 10% by weight of a polymerizable acidic monomer, up to 20% by weight Crosslinked rubber, up to 6% by weight of a thixotropic agent, up to 3% by weight of a tertiary amine initiator, up to 1% by weight of an inhibitor, and up to 5% by weight of a film time setting promoter at the edges and a catalyst component.
The composition of claim 3, wherein the first elastomeric material is a block copolymer of styrene and butadiene, and the crosslinked rubber is a copolymer of acrylonitrile and butadiene.
The composition of claim 4, wherein the block copolymer of styrene and butadiene has a styrene content of about 44-45%, and the acrylonitrile-butadiene copolymer has an acrylonitrile content of about 33%.
A composition used as a structural adhesive comprising a subcomponent comprising 10-30% by weight of the first elastomeric material, 5-20% by weight of the second elastomeric material, 40-65% by weight of the monomer representing the methacrylate ester, up to 10% % Polymerizable acid monomer, up to 10 wt% phosphate ester, 2-15 wt% crosslinked rubber, up to 5 wt% tertiary amine initiator, up to 5 wt% deformation time promoter at the edges, up to 2 wt% .% Inhibitor, and up to 10% by weight of a thixotropic agent and a catalytic component.
The composition of claim 6, wherein the polymerizable acid is isophthalic acid.
A composition used as a structural adhesive comprising a subcomponent comprising 15-35% by weight of a first elastomeric material which is a block copolymer of styrene and butadiene, 45-65% by weight of a monomer representing a methacrylate ester, up to 10% % Of a polymerizable acidic monomer that is isophthalic acid, up to 10% by weight of a phosphate ester, 2-15% by weight of a crosslinked rubber which is a copolymer of acrylonitrile and butadiene, up to 5% by weight of a tertiary amine initiator, up to 5% by weight of the film setting time setting promoter at the edges, up to 10% by weight of the thixotropic agent, up to 20% by weight of the cohesive fracture promoter, up to 10% by weight of the plasticizer, up to 5% by weight of the antioxidant and up to 2% by weight of the inhibitor, and A catalytic component, wherein the cohesive fracturing mode promoter includes esterified rosin or talc.
The composition of claim 8, wherein the subcomponent comprises up to 10% by weight of isophthalic acid.
The composition of claim 8, wherein the cohesive failure mode promoter is a combination of extraction rosin and talc.
The composition of claim 10, wherein the subcomponent comprises 0.01-20% by weight of a cohesive fracturing mode promoter.
-
A composition used as a structural adhesive, comprising a subcomponent comprising
10-25% by weight of the first elastomeric material,
1-10% by weight of the second elastomeric material,
50-70% by weight of the methacrylate ester monomer,
Up to 10% by weight of the polymerizable acid monomer,
Up to 10% by weight of the phosphate ester,
2-15% by weight of cross-linked rubber,
Up to 5% by weight of a tertiary amine initiator,
Up to 5% by weight of the film setting time promoter at the edges,
Up to 10% by weight of a thixotropic agent,
Up to 20% by weight of the cohesive failure mode promoter,
And up to 2% by weight of the inhibitor and a catalytic component, wherein the cohesive fracturing regime promoter includes esterified rosin or talc.
The composition of claim 12, wherein the polymerizable acid monomer is isophthalic acid.
The composition of claim 12, wherein the first elastomeric material is polychloroprene, and the second elastomeric material is a copolymer of acrylonitrile and butadiene.
The composition of claim 12, wherein the cohesive failure mode promoter is a combination of extraction rosin and talc.
The composition of claim 12, wherein the subcomponent comprises 0.01-20% by weight of a cohesive fracturing mode promoter.
print version
Date of publication on November 20, 2006




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.