| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Technology market / Current models of the invention and / back / |
|
INVENTION
Russian Federation Patent RU2113450
MOISTURE curable hot-melt adhesive based on polyurethane
![]()
Name of applicant: Henkel KGaA (DE)
Name of the inventor: Roland Heider (DE)
The name of the patentee: Henkel KGaA (DE)
Address for correspondence:
Starting date of the patent: 1993.11.16
Glue for joining shoe upper to the sole comprises at least one polyurethane prepolymer that consists of 15 -. 35% by weight of polyisocyanate, 10 - 70 wt. % Polyalkylene glycol 5 -. 65% by weight of polyether compound with a glass transition temperature (-40) - (+50) o C. It is stable during storage under cooling at ambient conditions is immediate high initial bonding strength and high creep resistance.
DESCRIPTION OF THE INVENTION
The invention relates to IP-melt adhesive, particularly a moisture-curing hot-melt adhesive based on polyurethane, which can find application particularly in the shoe industry.
The term "curing by moisture fusible adhesive based on polyurethane" is meant a high degree of solvent-free adhesive having functional urethane groups, which at room temperature is a solid and after application in a molten form binds both physically with cooling, but also due chemical interaction of the isocyanate groups still present with moisture. Only after this chemical curing is accompanied by an increase in size of the molecule, the adhesive exhibits its final valuable properties.
Known moisture curable hot melt adhesive based on polyurethane, comprising a copolymer of a simple and complex polyesters and polyurethane prepolymer of at least one polyol, which may be a simple mixture of one and one polyester and at least one polyisocyanate. This polyurethane based adhesive may contain a tackifier agent and a stabilizer [1].
When applying this known hot melt adhesives in the footwear industry, for example for connection with the sole of the shoe upper and its technological properties, particularly creep resistance, they are unsatisfactory and, moreover, the initial strength is needed fast enough to adversely affect the power performance.
From the standpoint of the technical essence and attainable result to the present invention, the closest technical solution is curable by moisture fusible adhesive based on polyurethane, which comprises 5 - 90% of the urethane prepolymer of at least one polyisocyanate, at least one polyalkylene glycol and at least one composite polyether and 10 - 95% of polymer based on ethylenically unsaturated compounds, wherein the known melt adhesive contains reactive water [2].
The disadvantage of hot melt adhesive is that when used in the shoe industry, for example for connecting the shoe upper to the sole, can not satisfy the requirement of continuously reducing cycle times, since it is impossible to reliably ensure the high creep resistance and sufficient green strength.
The object of the invention is to provide a curable by moisture melt adhesive based on polyurethane, in particular, for the footwear industry, which provides sufficient initial strength and, above all, a high resistance poluzuchesti with constantly decreasing time working footwear production cycle. In addition, the ultimate strength has to be achieved in a reasonable time and other technological and consumer properties, in particular flexibility at low temperatures, must not deteriorate.
Said problem is solved by curing a moisture-fusible adhesive based on polyurethane, which comprises at least one polyurethane prepolymer of at least one polyisocyanate, at least one polyalkylene glycol and at least one compound polyether, in which according to the invention as said complex poliefirkglikolya take complicated polyether glycol with a glass transition temperature (- 40) - (+50) o C, wherein the prepolymer is composed of 15 - 35 wt% of polyisocyanate, 10 -. 70% by weight of a polyalkylene glycol and 5-65% by weight of the above polyether compound...
By the term "polyurethane prepolymer" is understood oligouretan containing isocyanate groups and which is intermediate in the production of crosslinked polyurethane. By the expression "at least" one polyurethane prepolymer is meant that the adhesive has at least one peak at a molecular weight distribution curve. As a rule, the number of these peaks corresponds to the number of specific formed prepolymers of which is the result of a purely physical mixture of hot melt adhesive based on polyurethane. The upper limit of the amount of prepolymers, for practical reasons, is 3.
By the term "polyisocyanate" means a low molecular compound with 2 or 3 isocyanate-I. Diisocyanates are preferred, and they may contain about 10 wt.% Trifunktsinalnogo isocyanate. With increasing content of trifunctional isocyanate, of course, it should be understood that undesired crosslinking can occur both in the preparation and the application of the fusible adhesive. In addition to aliphatic and cycloaliphatic polyisocyanates are taken into consideration, especially, aromatic isocyanates. Specific examples are: toluene diisocyanate, diphenylmethane diisocyanate, and mixtures thereof. Under diisocyanate understand and 4,4 and 2,4 '-difenilmetandiizotsianat. Preferably, the 2,4'-isomer should not exceed 50 wt.%. Preferably use one or two different polyisocyanate. Above all use of 4,4'-diphenylmethane diisocyanate. Mixture with its 2,4 'isomer content affects the unreacted diisocyanate, thermal stability, and the duration of preservation and the ability to reactivation of the adhesive film. The content of the polyisocyanate in the fusible adhesive is preferably 20 - 30 wt.%.
The term "polyalkylene glycol" is meant a linear polyether with two OH-groups. Preferably it has the formula HO- (RO) m -H, wherein R is a hydrocarbon radical having 2-4 carbon atoms. It may be a copolymer with a block copolymer or a random copolymer. Specific polyalkylene glycols are polyethylene glycol, polytetramethylene glycol and, especially, polypropylene glycol (R = -CH 2 (CH 3) -). Preferably only one type of polyalkylene glycol. But it is possible to use mixtures of two or three of polyalkylene glycols which differ in their average molecular weights or by the form of their structure.
The amount of polyalkylene glycol, in particular polypropylene, is preferably 15 - 35 wt.%.
Primarily interested in pure polypropylene. Its average molecular weight should, generally, be 250 - 1000, preferably 350 - 600, particularly preferably 400 - 450. (These values are obtained OH-definition). Outside this range there is a clear deterioration of useful properties. Among other things, they are a high initial strength (= strength before curing), high creep resistance (shape stability = gluing toward irreversible deformation under the effect diminishing forces after increasing the time) in good yield and properties at the operating temperatures of application.
For this purpose can be used depending on the circumstances and other polimerdioly outside the preferred range, for example, polyesterdiols with the same molecular weight or tetraethylene glycol in the same amount as the polypropylene. Preferably the amount of these polimerdiolov that are outside the preferred range, there should be no higher than the number of preferred polyalkylene glycol.
The term "polyester polyether" is meant a polyester with two OH-groups, preferably two terminal OH-groups. They are prepared in a known manner from a) aliphatic hydroxycarboxylic acids, or from b) aliphatic dicarboxylic acids with 6 - 12 carbon atoms, in particular linear diols with 4 - 8 carbon atoms.
Of course, it can be used and the corresponding derivatives, e.g., lactones, methyl esters or anhydrides. Specific starting materials include: 1,4-butanediol, 1,4-hexanediol, adipic, azelaic, sebacic acid and lactone. The acid component may contain up to 25 mol.% Other acids such as cyclohexane dicarboxylic, terephthalic and isophthalic acids. The glycol component may contain up to 15 mol.% Of another diol such as diethylene glycol, 1,4-cyclohexanedimethanol. Apart from the homopolymers of the above units are important, above all, copolyesters from the following units, or derivatives thereof:
1) adipic acid, isophthalic acid, phthalic acid, and butanediol.
2) adipic acid, phthalic acid and hexanediol
3) adipic acid, isophthalic acid, phthalic acid, ethylene glycol, neopentyl glycol and 3-hydroxy-2,2-dimethylpropyl-3-gidoksi-2,2-dimethylpropanoate and
4) adipic acid, phthalic acid, neopentyl glycol and ethylene glycol.
The copolyester from adipic acid, isophthalic acid, phthalic acid, and butanediol is partially crystallized and has high viscosity. It thereby provides a high initial strength. The copolyester from adipic acid, phthalic acid and hexanediol has low glass transition temperature and thus provides an improved cold flexibility.
Sophisticated polyether are solid or liquid. When they are solid, they preferably have an amorphous structure, but there may be little crystallized. Preferably the initial mixture consisting of a partially crystallized and amorphous polyesters. Of course, the crystallinity is expressed to a small extent so that it is ready fusible adhesive makes no noticeable turbidity. Melting point partially crystallized polyester is in the range 40 to 70 o C, preferably from 45 to 65 o C. The melting temperature means the temperature at which the crystalline portion melted substance. It is determined by differential thermal analysis on the main peak on the endotherm. Preferably, the partially crystallized polyether compound used polibutandiol with a molecular weight of about 3500 adipate, etc. around 50 o C.
The average molecular weight of the polyether compound (Mn) should be 1500 - 30,000, preferably 2,500 - 6,000. It is calculated from the OH-numbers. Molecular weight of the polyether is of some importance: with increasing molecular weight of the hot melt extrusion difficult and its penetration into the skin, while reducing the molecular weight of a fusible adhesive at room temperature is not strong enough.
Sophisticated polyether preferably has a glass transition temperature (Tc) in the range of (-40) - (+40 o C ). The glass transition temperature determined by DSC at 10 o C per minute heating rate in the second cycle as the midpoint stage.
Particularly suitable are polyether complex such polyether complex which have a glass transition temperature of (-40) - (+40) o C, a viscosity of from about 3,000 to about 30,000 MPa · c at 130 o C (Brookfield, RVDV II + Thermosel) and hydroxyl number from about 27 to 60.
Preferably a mixture of two to six, in particular two or four, with various polyether complex glass transition temperatures. At the same time, as already mentioned, at least one polyether complex should have a glass transition temperature below 0 o C and one - above 0 o C, and the glass transition temperature values differ by at least 10 o C, preferably at 30 o C. The content of polyether compound with a low glass transition temperature should be 30 - 100 wt%, preferably 50 -. 90% by weight based on the total amount of polyether complex..
The resistance of the hot melt creep is further improved by the addition of hydrocarbon resin. In this case it refers to petroleum resin, terpene resin or coal tar. They have generally a molecular weight below 2000. The preferred hydrocarbon resin is an aromatic modified hydrocarbon resin, terpene resin, such as poly-alpha-methylstyrene, rosin ester, coumarone / indene resin. Of course these substances act as tackifiers.
Their weight content in the fusible adhesive is 0.1 - 15, in particular 3 -. 10% by weight.
Furthermore, hot-melt adhesive of the invention may contain stabilizers, which must support the physical properties as much as possible, constant, particularly melt viscosity and coloration. This can be used for at least one of the following, as referred to in the examples of substances: phosphoric acid, phosphorous acid and toluene-sulfonyl isocyanate. It is advisable to take 0.01 - 0.5, preferably 0.01 - 0.1 wt% stabilizer-toluene sulfonyl isocyanate..
To accelerate the curing reaction can be added for the synthesis of the known polyurethane catalysts, e.g., olovodiorganicheskie compounds such as dibutyltin dilaurate or olovomerkaptanovye compound. The amount of these compounds is not higher than 1.5, preferably 0.5 - 1% by weight of the prepolymer..
Hot melt adhesive according to the invention preferably contains no solvent. The term "solvent" is meant inert organic compound with m. Bales. not more than 200 o C. Hot melt adhesive according to the invention preferably does not contain inert fillers, such as alumina, carbonates, and titanium dioxide.
The fusible adhesive according to the invention, the ratio of reactive groups NCO: OH of 1.1: 1 - 2: 1, preferably 1.15: 1 - 1.5: 1. For specific compositions be selected such ratio NCO: OH, to a suitable hot-melt adhesive had a molecular weight, i.e. molecular weight should be sufficient to provide good initial strength, on the other side, it must be so low that the viscosity and without solvent has a sufficiently low as in the manufacture and in use. Furthermore, hot-melt adhesive should have a minimum even 0.5 - 3 g, preferably 1.0 - 2 g of free NCO-groups per 100 grams of hot melt adhesive to provide sufficient moisture curing. The content of NCO-groups determined by titration.
The melt viscosity of hot melt adhesive based on polyurethane according to the invention is preferably in the interval 20 - 100 Pa · c. Melt viscosity measured at 130 o C according to Brookfield, the preheated sample was 15 minutes at 130 o C and then fixed viscosity.
The type and amount of each component should be selected so that they are consistent with each other. An indication of such matching is that in the DSC -diagram polyurethane prepolymer predominantly observed only one glass transition temperature (Tg). This measurement is carried out at the second cycle at a temperature elevation rate of 10 o C per minute.
The polyurethane prepolymer of the invention can be manufactured as a single stage or in several stages. stirred together by implementing a multistage process, for example, is first reacted with the polyisocyanate separately with and polyalkylene polyether complex and then reaction products. It is also possible first to interact only with the polyisocyanate or only polyalkylene polyether with a complex and then continue the transformation of treating a precursor, in the presence of all other reagents.
Preferably, however, to produce a polyurethane prepolymer according to the invention for one-step process. For this first mixed and complex polyalkylene polyether and then dehydrated for 60 minutes at a temperature of 110 - 130 o C under vacuum. After cooling the mixture to 90 o C was added polyisocyanate. The reaction mixture was again heated to 110 - 130 o C. If no catalyst is added, the process continues, in general, about 60 minutes before the time when the interaction under vacuum almost ends, i.e. OH-groups no longer be determined or its amount is up to 2 g per 100 g of prepolymer or, and when the viscosity reaches the desired value.
If not necessary additives are introduced into the reaction mixture during the synthesis of the polyurethane prepolymer, they should be added to the resulting prepolymer and homogenize them therein.
As reactive polyurethane prepolymer containing NCO-groups, the fusible adhesive based on polyurethane is sensitive to atmospheric moisture. Therefore, during storage it must be protected from moisture. To do this, it is advisable to pack it in a sealed, dry and moisture-proof container of aluminum, tinplate or multilayer foil.
Hot melt adhesive according to the invention is characterized essentially by the following valuable properties:
It does not contain solvent. The concentration of unreacted MDI is less than 2.0 wt.%, Preferably below 1.0 wt.%.
It is stable during storage, i.e. as there was no separation. At the application temperature, e.g., 170 o C, it is relatively stable, i.e. the melt viscosity is reduced in the area of ± max. one-third, preferably one fifth of the initial value for 4 hours.
It can be easily applied at a temperature of 110 - 180 o C in the form of easy-flowing melt.
It wets the skin or the rubber sufficiently and even penetrates relatively deep into the fibrous material.
Before final curing is enough time to accommodate the bonding seam.
Upon cooling to ambient conditions occurs immediately with high initial bonding strength and high creep resistance. The surface of the adhesive layer is not sticky after cooling.
During the normal storage time semifinished shoe reached acceptable strength values.
bonding locations are flexible, including at low temperatures.
Place bonding transparent.
Place bonding after curing has a high resistance against the action of water.
Due to these properties, the hot melt is useful according to the invention is preferably suitable for use in the shoe industry, in particular in machines for the application which are included in the technological production line shoes that do not contain pre-crosslinking step by the action of steam, or respectively drying tunnel.
The concept includes any kind of footwear products, including not only the finished marketable products, and intermediate and semi-finished blanks.
The notion of sole includes all external surfaces of the soles, including the heels.
Hot melt adhesive according to the invention is particularly suitable for bonding soles to shoe uppers, and, moreover, for the hardening materials subjected to stress and effort to bond skins.
The adhesive is preferably applied to the machine application thickness 0,05-0,7 mm. After the application layer and the fusible layer before the compression of the joint surfaces to be bonded and the melt adhesive can be cooled and stored semi-finished blank, wherein curing occurs. Then, before compression joint preform is reheated to a temperature of 110-180 o C, when the adhesive was applied to only one workpiece or to 50-100 o C, when the adhesive was applied to both the preform.
Curing can be carried out under various conditions. In particular, it is carried out under the action of moisture from the air. To this end, the relative humidity should be above 25% at 20 o C. Under these conditions, the curing takes place for at least 24 hours. Environmental conditions may be inferior, for example, in 20 ± 5 o C. In any case, the relative humidity must not be lower than 10%, so that the curing duration was 3-7 days.
Application of a fusible adhesive according to the invention in the shoe industry has the following advantages:
It enables to use the system, which is directly involved in the process line.
Due to the high creep resistance and initial strength, it can significantly improve performance.
The strength of adhesion to the skin unexpectedly high.
In some cases it is sufficient sided coating, for example, in the case of PU -podoshv.
The invention is explained in more detail by the following examples.
A) Starting materials:
The polyester A is a partially-crystallized complex glycol copolyester of isophthalic acid, butanediol, adipic acid and dimethyl phthalate. The polyester A has a molecular weight of about 3500, a hydroxyl number of 27 - 34, determined according to DIN 53-240, the glass transition temperature of about 20 o C, determined by DSC, and a viscosity at 100 o C of about 30,000 MPa · s and at 130 o C about 5000 mPa.s determined by a Brookfield viscometer (LVT4).
Polyester B is a partially-crystallized copolyester glycol from dimethyl phthalate, adipic acid and hexanediol. It has a molecular weight of about 3500, a hydroxyl number of 27 - 34, determined according to DIN 53240, a glass transition temperature of about 40 o C, determined by DSC, and a viscosity at 130 o C of about 3,000 mPas determined with a Brookfield viscometer (LVT4).
The polyester is a solid, amorphous copolyester glycol of neopentyl glycol isophthalic acid, adipic acid, phthalic acid and 3-hydroxy-2,2-dimethylpropyl-3-hydroxy-2,2-dimethylpropanoate. It has a molecular weight of about 3500, a hydroxyl number of 31 - 39, determined according to DIN 53240, a glass transition temperature of about +30 o C, determined by DSC, and a viscosity at 130 o C of about 30,000 mPas determined with a Brookfield viscometer (LVT4) .
Polyester D is a solid, amorphous copolyester of neopentyl glycol, ethylene glycol, adipic acid and phthalic anhydride. It has a molecular weight of about 2000, a hydroxyl number of 50 - 60, determined according to DIN 53 240, the glass transition temperature of about +10 o C, determined by DSC, and a viscosity at 80 o C of about 70,000 MPa · s and at 130 o C of about 5000 mPas determined with a Brookfield viscometer (LVT4).
Polyester D is very poorly crystallized, saturated copolyester-glycol with a viscosity index of 77 - 83 cm 3 / g, determined according to DIN 53 728, a softening point of 102 - 110 o C, determined in accordance with DIN 52011, glass transition temperature of about 12 o C determined by DSC, and a melt viscosity at 180 o C 65 - 75 Pa · s and at 200 o C 35 - 40 Pa · s, determined by rotational viscometer (Platte / Kegel). This polyester has a molecular weight of about 25,000 as determined by the number of OH.
Polyester E is a solid amorphous copolyester glycol having a molecular weight of about 3500, a hydroxyl number of 27 - 34, determined according to DIN 53240, a glass transition temperature of about 20 o C, determined by DSC, and a viscosity at 130 o C of about 7000 mPa · s determined by a Brookfield viscometer (LVT4).
The polyesters are commercial products company Hyuls AG, Germany.
Polypropylene glycol has a molecular weight of about 425 and is a product of Miles Inc. company., USA.
Tackifying agent is a polymer of beta-pinene resin manufactured by Herkules Inc., USA.
Hydrocarbon resin is a product of the company Arizona Kemikel Co., USA.; it is used as an amplifier to increase tackiness and creep resistance.
Difenidmetan-4,4'-diisocyanate (MDI) is a product of the firm Miles Inc., USA.
B) Preparation:
For polyurethane adhesive the following compound number in Tables polyether or polyether mixture and polypropylene glycol complex, but also, if necessary, polymeric beta-pinene resin was dehydrated for 60 minutes at a temperature of from about 110 to about 130 o C under vacuum. After cooling to about 90 o C the mixture is introduced in a specified number of diphenylmethane-4,4'-diisocyanate (MDI) and reacting at a temperature of from about 110 to 130 o C under vacuum for about 60 min. After the reaction, the prepolymer is charged into a moisture-proof container.
B) Tests
Viscosity measured with a Brookfield viscometer termoyacheistym: after 15 minutes heating tube with molten polyurethane at these temperatures fixed viscosity. Polyurethane melt stability was determined by measuring viscosity increase for two hours, generally at 130 o C.
The peel strength is determined on specimens of SKS (styrene butadiene rubber), a width of 2.5 cm and 10-12 cm long. The strips immersed for 5 seconds in the primer solution, and then dried for 30 minutes in an oven at 100 o C. The material of the top shoes are either leather or fabric coated with a polyurethane coating, the surface of which a known manner to give some degree of roughness. Glue is applied to a layer about 0.5 mm thick onto the heated and primed sample of SCS. The heated shoe upper is pressed against the pattern of first SCS hand, then for 1 min in a press under a pressure of 7 - 14 bar (100 - 200 psi). Alternatively, the glue is applied to the surface of both heated, compressed first manually, and then for about 1 minute in a press under a pressure of 7 - 14 bar (100 - 200 psi). After a specified period of time measured peel strength for samples that are fed to the tensile testing machine "Instron" and pulled apart at a rate of 1.25 cm / min. Under the binding strength in the dry state understand the peel strength in the cured state after 7 days.
Creep resistance is determined by attaching a 500 g load to the top of the material neproklennoy shoe samples used for testing the peel strength. Creep resistance is measured in mm / h. The rating corresponds well to the value below 10, preferably below 5 mm / h. After curing the adhesive test is performed at various temperatures to evaluate the thermal stability and adhesive.
The following examples 1 - 5 (.. See Table 1) show the effect on the amount of polyether compound early strength (peel strength = 5 min).
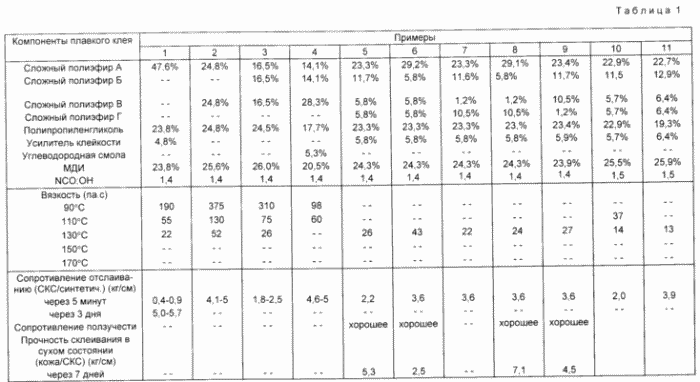
Examples 6 - 11
(Invention)
example 12
This example demonstrates the preferred hot melt adhesive compositions of the invention (see. Table. 2).
Initial strength is determined on specimens in the form of two strips of 12.5 cm length and 2.5 cm width, made of NBR / NBR (butadiene rubber), which is preheated to 80 - 100 o C. Glue is applied at a temperature of about 180 o C in both strips and squeeze them together for about 1 minute at 7 bar (about 100 pounds per square inch). It should be noted that such compression strips depending on the apparatus, and possibly a lower pressure. The above examples of the strips comprise a substrate of NBR nitrile rubber with a Shore hardness of 80, they are obtained from the Institute for testing and research for making shoes, Germany.
The initial strength was determined at the speed of 12.5 cm / min (5 inches per minute). The values obtained in pounds per linear inch are converted to kg / cm with an index of 5.61.
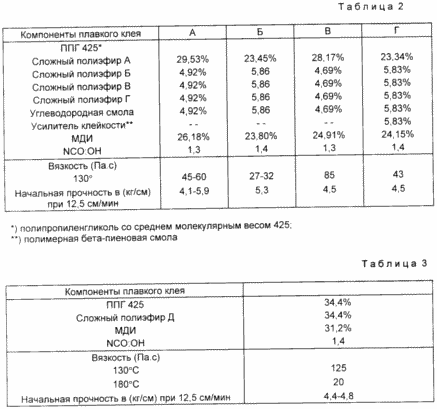
example 13
This example illustrates that when using high molecular weight polyesters can be prepared fusible adhesives based on polyurethane with satisfactory properties (see. Table. 3).
CLAIM
A moisture-curing hot-melt adhesive based on polyurethane, comprising at least one polyurethane prepolymer of at least one polyisocyanate, at least one polyalkylene glycol and at least one polyether compound, wherein said compound as said charge polyether polyether complex with a glass transition temperature of -40 to 50 o C, wherein the prepolymer is composed of 15 - 35 wt% of polyisocyanate, 10 -. 70% by weight polyalkylene glycol and 5 -. 65% by weight of said polyether compound..
Adhesive according to claim 1, characterized in that it comprises a resin in an amount of 0.1 - 15.0% based on the total weight of the hot melt adhesive.
Adhesive according to claim 1, characterized in that it comprises the stabilizer in an amount of 0.01 - 0.5% based on the total weight of the adhesive.
Adhesive according to claims 1 - 3, characterized in that the prepolymer comprises at least two different polyether glycol complex glass transition temperatures, one complex polyether has a glass transition temperature below 0 o C, and the other - higher than 0 o C, and glass transition temperature values vary between a minimum 10 o C.
Adhesive according to claims 1 - 4, characterized in that the prepolymer of DSC diagram is observed only one glass transition temperature.
The adhesive of claim. 1 - 5, characterized in that its melt viscosity at 130 o C of 10 - 300 Pa · c.
print version
Publication date 03.12.2006gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.