| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Technology market / Current models of the invention and / back / |
|
INVENTION
Russian Federation Patent RU2225360
MALAKHIT AND METHOD FOR PRODUCTION THEREOF
![]()
Name of applicant: Sokolov Valeriy
Name of the inventor: Sokolov VV .; Petrov TG .; Kopeikin PF
The name of the patentee: Sokolov Valeriy
Address for correspondence: 197136, Saint-Petersburg, 88 AY, pat.pov. O.V.Novoseltsevu, Identification No 65
Starting date of the patent: 2003.02.25
The invention relates to the production of artificially grown stones for jewelery, arts and crafts, and chemical industries. Invention: Preparation of polycrystalline malachite given thickness did not differ in their properties from the best varieties of natural malachite. Malachite represents the basic carbonic copper, zinc, and impurities in a compound based on Zn +2 in an amount of from 0.2 to 0.9 wt. %. Malachite zinc compound comprises a basic zinc carbonate Zn 2 [CO 3] (OH) 2 or ZnCO 3 · Zn (OH) 2, and contain the impurity Fe 2 O 3, Na 2 O and residual ammonium ion into intercrystalline space. . The ratio of components in malachite, m ac%: Cu 2 [CO 3] (OH) 2 or CuCO 3 · Cu (OH) 2 99,509-99,15; Zn +2 0,48-0,58; Fe 2 O 3 0.1-0.2; Na 2 O 0,01-0,1. The method includes receiving malachite evaporating solution of a basic copper carbonate and basic zinc carbonate in an aqueous solution of ammonium carbonate. The ratio of copper to zinc is evaporated in the solution is from 0.018 to 0.09, preferably from 0.037 to 0.049 g per gram of Zn +2 Cu +2. Evaporation of the basic copper carbonate and basic zinc carbonate in the ammonium carbonate aqueous solution is carried out by condensing the resulting vapor mixture by evaporation of NH 3, CO 2 and H 2 O and obtain an aqueous solution of ammonium carbonate. ammonium carbonate solution used to dissolve the basic copper carbonate and evaporated to obtain the feed solution of copper carbonate to basic aqueous solution of ammonium carbonate.
DESCRIPTION OF THE INVENTION
The invention relates to the industrial production of artificially grown stones for jewelery, arts and crafts, and chemical industries.
The invention may find application in the manufacture and restoration of jewelry, fashion jewelry, souvenirs, objects of arts and crafts, museums interiors of apartments and buildings.
Malachite is a mineral chemical composition from the class of carbonates of Cu 2 [CO 3] (OH) 2 or CuCO 3 · Cu (OH) 2, containing 71,9% CuO (Cu 57,4%) , 19,9% CO 2, 8,2% H 2 O and up to 10% impurities in the form of CaO, Fe 2 O 3, SiO 2. Practically insoluble in water, readily soluble in mineral acids, is thermally unstable and, at temperatures above 100 o C decomposes to tenorite (CuO). It crystallizes in the monoclinic system, crystals are rare and have a needle or prismatic shape. Are common crypto and kidney-shaped fine-grained sinter crust, stalaktitopodobnye units, rhythmically banded with radial-fibrous structure.
Color natural dense malachite from bright green with a variety of color transitions to dark, sometimes brown-green. The color change on different regions and layers of malachite creates in sections and polished planes quirky drawing, giving beauty products from malachite. Gloss units at Silky (plush malachite), velvety, dim, in crystal - diamond, rolling in a glass. Hardness on Mohs Mohs 3.5-4.0; density of 3900-4100 kg / m 3.
In nature, malachite occurs in the near-surface zone of oxidation of sulfide copper ores, which explains the presence of impurities in the natural malachite in the form of CaO, Fe 2 O 3, SiO 2. Large accumulations of dense malachite are very rare and are formed by replacement of limestone copper sulphate solution in the oxidation zone of large copper deposits. Usually found in small quantities in a dispersed state in the form of raids, gouges, small clusters, earthy masses mixed with other supergene minerals and only occasionally encountered dense concentrations of malachite weighing several tons to tens of tons (50 tons Mednorudnyansk, Nizhny Tagil, Gumeshevskie mines in the Urals). [1]
Thick, zonal-concentric wandering malachite in the form of fairly large masses of great value as a beautiful ornamental stone is used for jewelry and decorative art products (inserts, beads, table tops, vases, columns lining, etc..).
There are large deposits of malachite in Zaire, South Australia, Kazakhstan and the United States.
Deposits of malachite in the Urals (Mednorudnyanskie Gumeshevskie and mines) are currently developed. In this connection there is an actual problem of development of malachite growing technology in industrial environments, the same or even superior in the quality indicators of natural malachite.
Known methods of producing synthetic gemstones materials, consisting of the crystallization of salt melts or high-water solutions [2]. However, to obtain data malachite methods are not suitable as malachite due to thermal instability decomposes at 100-110 o C without melting, and substantially insoluble in water.
Known methods of preparing single crystals of malachite under low temperature hydrothermal synthesis of [3].
A method for manufacturing a synthetic malachite as individual particles and their co-precipitation with a small amount of bismuth diffused uniformly used as nuclei for the subsequent growth at elevated temperatures and subsequent conversion to acetylene copper complex used as a catalyst etilinirovaniya [4].
Agglomerates of malachite crystals are known and their preparation containing 7.1% (BiO) 2 and 3 SuSO 0,5-3,5% SiO 2, having a mean size of 15 microns, used as catalysts in the chemical industry. [5]
And known production method or malachite malahitopodobnyh products comprising natural malachite grinding to particles of 10-100 microns, powder distribution in the transparent lacquer, stain them manufactured items, and drying the coating on the surface of masks or patterns which reproduce natural texture malachite [6].
A method of producing polycrystalline malachite comprising dissolving copper carbonate in an aqueous solution of ammonium carbonate containing an equal molar proportion of ammonium ions and carbonate, followed by evaporation of the solution under heating, resulting in a loose sediment polycrystalline malachite [7]. The disadvantage of this method obtained by the malachite is weak fusion between individual crystals and polycrystalline spherulites in the resulting sediment, its high porosity and low mechanical strength (after drying the residue is easily pounded fingers), which makes it unsuitable for gemstones purposes. Another disadvantage of this method is the monotony of sludge resulting malachite, having a pale green color, in contrast to the dense polycrystalline aggregate natural malachite gemstones whose species are characterized by alternating light-green and dark green stripes or strata.
The common disadvantage of the methods described above is the inability to obtain a dense material, similar in its characteristics to natural malachite and suitable for use in jewelry and ornamental purposes.
The closest the technical essence and achieved using the technical result (prototype) is developed by the authors earlier synthetic gemstones, malachite, and its production method [8].
Synthetic gemstones malachite prototype is a polycrystalline aggregate containing basic carbonic copper Cu 2 [CO 3] (OH) 2 and as impurities Fe 2 O 3 and Na 2 O, at a ratio of components, wt.%:
Cu 2 [CO 3] (OH) 2 - 99.99 - 99.5
Impurities - 0.01 - 0.50
Synthetic malachite prototype has a Micro - 216-390 kg / mm 2, density of 3.9-4.1 g / cm 3, Mohs hardness - 4.0 maximum reflectance spectrum synthetic malachite - 490-525 nm.
A characteristic feature of synthetic malachite prototype method for its preparation is by dissolving basic copper carbonate in an aqueous solution of ammonium carbonate containing a molar excess of ammonia in 1.5-8 times molar content relative to carbon dioxide, and subsequent evaporation of the solution under heating to form a polycrystalline machine, whereby synthetic malachite intercrystalline space contains residual ammonium ion, and solution-evaporation basic copper carbonate in an aqueous solution of ammonium carbonate with an excess ammonia is carried out at a temperature of 40-95 o C, preferably at a temperature of 60-80 o C, and the basic solution evaporated copper carbonate in an aqueous solution of ammonium carbonate with an excess ammonia is carried out at a variable rate.
By way of prototype fails in contrast to other previously known methods to obtain a polycrystalline dense malachite, suitable for use in jewelry and ornamental purposes, but synthetic malachite prototype has several technical flaws.
In particular, disadvantages and malachite synthetic method of preparation of the prototype are:
- insufficient mechanical strength of the synthetic malachite, which leads to great losses of material and, consequently, to the deterioration of the technical and economic indicators of gemstones products;
- the inability to obtain a sufficiently large thickness malachite due to non-union between the individual layers are grown malachite in the charge of the evaporator, which limits its use in products of arts and crafts;
- formation upon evaporation of the basic carbonate of copper in an aqueous solution of ammonium carbonate vapor mixture of carbon dioxide, ammonia and water vapor, which makes it necessary, from the viewpoint of environmental safety, the use of special devices for disinfection released during evaporation carbonic acid solution and the ammonia gas, with ammonia in toxicity a hazardous substance, refers to the 4th group of hazardous substances according to GOST 12.1.07-76 and has a maximum permissible concentration (MPC) of 20 mg / m 3;
- evaporating the solution according to known technology leads to irreversible loss of ammonia and carbon dioxide, the major components of technological process of evaporation.
OBJECTIVES AND OBJECTS OF THE INVENTION
The main technical problem (is not allowed to date Inventive Problem Solving), restraining the expansion of industrial production and widespread use of malachite in gemstones, decorative and artistic purposes, it lies in the fact that the hitherto known methods are environmentally hazardous and known methods can be obtained malachite insufficiently high strength (micro-hardness), and in the fact that known methods can not obtain a sufficiently large thickness of material from a non-union between the individual layers of malachite generated during recharging evaporator.
The aim of the invention (the required technical result is achieved by using the invention) is to be able to eliminate the above drawbacks, while ensuring the possibility of obtaining polycrystalline malachite almost any desired thickness, its properties do not differ, and in some respects and superior properties of the best varieties of natural malachite.
SUMMARY OF THE INVENTIONS
The target and the required technical result is achieved by the invention in that the invention comprises malachite zinc compound based on Zn +2 in an amount of from 0.2 to 0.9 wt.%.
Thus malachite of the invention comprises a zinc compound as the main carbon zinc Zn 2 [CO 3] (OH) 2 or ZnCO 3 · Zn (OH) 2 and impurity malachite contain Fe 2 O 3, Na 2 O and residual ammonium ion in intercrystalline space.
Thus malachite of the invention contains a basic carbonic copper Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2, a zinc compound based on Zn +2 and impurities at the following ratio, wt.%:
Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2 - 99.509 - 99.15
Zn +2 - 0,48 - 0,58
Fe 2 O 3 - 0.1 - 0.2
Na 2 O - 0,01 - 0,1
Microhardness malachite of the invention is 420-440 kg / mm 2, malachite wear resistance compared to natural wear malachite is 160-180%, polishing with respect to polishability natural malachite is 105-150%, the density of 4.0-4.1 g / cm3, a Mohs hardness of 4 and a maximum of reflection spectrum 500-535 nm.
Malachite of the invention is a polycrystalline aggregate obtained by evaporation of the solution of a basic copper carbonate and basic carbon dioxide in an aqueous solution of zinc ammonium carbonate comprises alternating contrasting light green and dark green layers malachite surface in reflected light exhibits plush moire effect.
The target and the required technical result when using the invention is achieved by the fact that a process for preparing the malachite comprising evaporating solution of a basic carbonate of copper in an aqueous solution of ammonium carbonate, the inventive solution is evaporated basic carbonic copper Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2 and the main carbon zinc Zn 2 [CO 3] (OH) 2 or ZnCO 3 · Zn (OH) 2 in an aqueous solution of ammonium carbonate at a ratio of zinc to copper in the solution was evaporated of from 0.018 to 0, 09, preferably from 0.037 to 0.049 g per gram of Zn +2 Cu +2.
When this solution was evaporated basic copper carbonate and basic zinc carbonate in aqueous ammonium carbonate solution is prepared by dissolving basic copper carbonate and basic zinc carbonate in the ammonium carbonate aqueous solution with excess ammonia in the molar content of 1.5-8 times its molar content of carbon dioxide, evaporating the solution of a basic copper carbonate and basic zinc carbonate in the ammonium carbonate aqueous solution is carried out at a temperature of 40-95 o C, preferably at a temperature of 60-80 o C.
Evaporation of the solution of the basic carbonate of copper and basic carbon zinc in an aqueous solution of ammonium carbonate is carried out at a variable rate to enable the crystallization of malachite with alternating contrasting layers, for example, light green and dark green, and the rate of solution evaporation in growing the next layer of malachite with contrast color transition changing by at least 1.2 times as compared with the evaporation rate of the previous layer during crystallization until a predetermined malachite malachite layer thickness.
Additionally evaporating solution of a basic carbonate of copper and basic carbonate of zinc in aqueous ammonium carbonate is carried out with condensation produced by evaporating a solution of the steam-gas mixture of NH 3, CO 2 and H 2 O to obtain an aqueous ammonium carbonate solution that is used to dissolve basic carbonic copper and base carbon zinc and receiving supplied to the evaporating solution of a basic carbonate of copper and basic carbonate of zinc in an aqueous solution of ammonium carbonate, and evaporating the solution basic carbonic copper and basic carbonate of zinc in an aqueous solution of ammonium carbonate and condensation formed by evaporating the vapor-gas mixture of NH 3, CO 2 and H o 2 is carried out in a closed loop, formed by the condensation evaporating solution of a basic copper carbonate and basic zinc carbonate in aqueous ammonium carbonate solution of NH 3 gas mixture, CO 2 and H 2 o is carried out at a temperature of 30-55 o C, preferably at a temperature of 40-50 o C.
The result is a malachite of the invention with properties and quality as described above.
The target and the required technical result when using the invention is achieved by the fact that a process for preparing the malachite comprising evaporating solution of a basic carbonate of copper in an aqueous solution of ammonium carbonate, the inventive solution-evaporation basic carbonate of copper in an aqueous solution of ammonium carbonate is carried out with condensation formed by evaporating the vapor-gas mixtures of NH 3, CO 2 and H 2 O and form an aqueous ammonium carbonate solution which is used to dissolve the basic copper carbonate and evaporated to obtain the feed solution of copper carbonate to basic aqueous solution of ammonium carbonate.
In this solution-evaporation basic carbonate of copper in an aqueous solution of ammonium carbonate, condensation formed by evaporating the vapor-gas mixture NH 3, CO 2 and H 2 O, dissolved basic carbonate of copper in an aqueous carbonate solution and ammonium dissolving basic carbonic copper and getting fed to the evaporation of the solution substantive copper carbonate in an aqueous solution of ammonium carbonate carried out in a closed cycle until a predetermined thickness of malachite layer.
Evaporation of the solution of a basic copper carbonate in an aqueous solution of ammonium carbonate is carried out at a temperature of 40-95 o C, preferably at a temperature of 60-80 o C and condensing the resulting vapor mixture by evaporation of NH 3, CO 2 and H 2 O is carried out at a temperature of 30-55 o C, preferably at a temperature of 40-50 o C.
In addition the solution was evaporated basic carbonic copper Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2 in an aqueous solution of ammonium carbonate with the addition of basic carbonate of zinc Zn 2 [CO 3] (OH) 2 or ZnCO 3 · Zn (OH) 2 at a ratio of copper to zinc in the solution ranging from 0.018 to 0.09, preferably from 0.037 to 0.049 g per gram of Zn +2 Cu +2 and condensing the resulting vapor mixture with NH 3, CO 2 and H 2 O aqueous ammonium carbonate solution used to dissolve the basic copper carbonate and basic zinc carbonate to obtain a solution of a basic copper carbonate and basic zinc carbonate in aqueous ammonium carbonate in a closed loop to obtain malachite of the invention described above.
REALIZATION EXAMPLES OF THE INVENTION
Validation of the inventions, the inventions possibility of industrial implementation and the possibility of practical realization of the required technical result is confirmed by the following examples of the invention.
When growing malachite powder used according to the invention the basic carbonic copper Cu 2 (OH) 2 CO 3 or SuSO 3 · Cu (OH) 2 in accordance with GOST 8927-79, powdered basic zinc carbonate Zn 2 (OH) 2 CO 3, ZnCO 3 · Zn (OH) 2 TU 6-09-3676-77, ammonium carbonate (NH 4)2 CO 3 in accordance with GOST 3770-78 and 25% aqueous ammonia solution of NH 4 OH in accordance with GOST 3760-79, distilled water.
example 1
Basic carbonic copper Cu 2 (OH) 2 CO 3 dissolved in a solution of ammonium carbonate (MN 4)2 CO 3 containing a molar excess of ammonia NH 3 with respect to the molar content of carbon dioxide CO 2.
The molar ammonia content relative to carbon dioxide molar contents to the conditions of Example 1.5.
The mixture was stirred until complete dissolution of the basic copper carbonate. The solution is analyzed for copper cation and applied to the main zinc carbonate solution in the amount of 0.018 grams of zinc per gram of copper contained in the solution. Evaporation of the solution was carried out at 40 o C.
For alternating light-dark green bars evaporation process was carried out with a variable speed, variable change in the range of 1.2 times with respect to the evaporation rate at the preceding stage receiving light or dark bands (layers). evaporation process continues until no more ammonia vapor. Termination of ammonia vapor indicates complete decomposition mednokarbonatnoammiachnyh complexes formed in the process of dissolution of the basic carbonate of copper in ammonium carbonate solution, which leads to the formation of a dense polycrystalline aggregate basic copper carbonate, which is a synthetic gemstones malachite. After the end of the process the remaining aqueous residue is removed from the synthetic malachite and analyze it for compliance with the parameters of the standard sample of natural malachite presented in the ICDD database, 41-1390, was analyzed for zinc content and micro-hardness was measured on samples of the material mikrotverdometre PMT-3, adjust for standard procedure. The results of measurement and analysis conditions prepared in this example are shown in FIG. Wear resistance was measured by a standard technique [Kartashov IN
et al. Treatment of parts free abrasives in vibrating tanks.
& Publishing association Vyshcha school.
- Kiev.
- 1975, pp 42-52] in a vibratory machine in a mixture of natural products and the Ural malachite malachite of the invention..
Wear resistance was determined the weight of the products before the trial and at the end of the process. The results are shown in Table .
examples 2-12
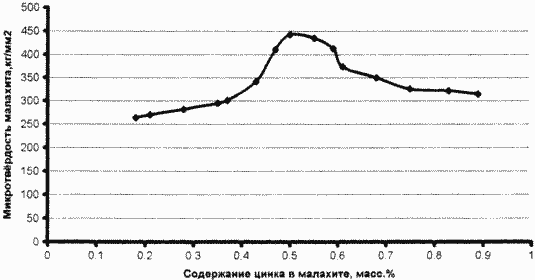
Examples 2-12 conditions similar to those of Example 1, but the proportion of zinc Zn +2 contributed salt basic zinc carbonate was respectively for Example 2 - 0.028, for example 3 - 0.036, for example 4 - 0.043, for example 5 - 0.047, for example 6 - 0,050, for example 7 - 0.055, for example, 8 - 0.06, for example, 9 - 0.062, for example 10 - 0.07, for example 11 - 0.08, for example, 12 - 0.09 g Zn + 2 per gram of Cu +2 in solution. For each sample, grown on the examples given conditions malachite of the invention, determined the weight content of zinc Zn +2 and microhardness in comparison with micro-hardness of natural Ural malachite. The results are plotted in FIG.
example 13
A solution of the basic copper carbonate with the addition of basic zinc carbonate on the conditions of Example 1.
Next, a batch of the powdered basic carbonate of copper and zinc basic carbonate with an optimal ratio of zinc and copper. The amount of the charge is calculated on the basis of specified sizes and volumes grown malachite. The charge loaded into the evaporator and sealed. Evaporation of the solution was carried out at a temperature of 40-95 o C, preferably at a temperature of 60-80 o C with variable speed and condensing the vapor phase produced was carried out at 30 o C.
Resulting in the condensation of ammonium carbonate solution is sent to dissolve the charge to form a basic solution was evaporated copper carbonate and basic zinc carbonate. The resulting solution is automatically dosed in the evaporation zone and polycrystalline grown malachite. cultivation process was carried out in a closed loop in the vehicle, preventing from hazardous gases in the working area. This was accompanied by reuse of components, especially ammonia and carbon dioxide taken for preparing the starting solution. At the end of the process, ie the complete dissolution of the charge was determined by the process of time, the growth rate of malachite and its geometrical dimensions. The results are shown in the table.
Examples 14, 15, 16
Terms of the similar conditions as in Example 13, but the condensation temperature was changed in the following sequence:
- for example 14, the condensing temperature of 40 o C;
- for example 15, the condensing temperature of 50 o C;
- for example 16, the condensing temperature of 60 o C.
The results are shown in the table.
Past studies have shown rentgenodifraktometricheskie identity radiographs of natural and malachite of the invention.
Almost all of the optical constants of the invention are grown malachite similar optical constants of natural malachite.
As natural malachite grown malachite of the invention in the reducing flame melts and gives bead copper. In the gas burner flame malachite stain blue. When heated in a glass tube malachite allocates water and blackens, dissolved in hydrochloric acid with a hiss.
The invention allows to obtain almost any desired thickness grown malachite by organizing process in a closed cycle condensing gas mixture liberated during the evaporation of the solution basic carbonic copper and basic carbonate of zinc in aqueous ammonium carbonate or basic carbonate solution of copper in an aqueous solution of ammonium carbonate and using formed by the condensation of ammonium carbonate solution to dissolve the next portion of the reagent mixture evaporated.
Thus, the invention allows to obtain with malachite physicochemical properties characteristic of natural malachite consumer quality malachite of the invention differs from the natural increased microhardness and high wear resistance, due to a lower content qualitative composition and other impurities.
INFORMATION SOURCES
Great Soviet Encyclopedia (BSE), s.276.
NI Kornilov, Solodova YP Jewelry stones. - M .: Nedra, 1987, pp. 259-276.
Ruszala F., Kostiner E. The hydrothermal synthesis of single crystals of ozurite and malachite. J. Cryst Growth. 1974/26, 1, s.155-156.
US Patent No. 4107082, B 01 J 27/20, 15.08.78.
US Patent No. 4536491, B 01 J 21/20, C 04 C 33/04, 20/08/85.
Patent EP 0856363, B 05 D 5/05, B 44 F 9/04, 1998.08.05.
Chirvinsky PN Selected works. Artificial obtaining minerals in the XIX century. - M .: Nauka, 1995, p.278 and 279.
Russian patent 2159214, C 01 G 3/00, publ. 20.11.2000, BI 32 (prototype).
CLAIM
Malachite containing basic carbonic copper and impurities, wherein the zinc compound comprises malachite.
Malachite according to claim 1, wherein the zinc compound contains malachite based on Zn +2 in an amount of from 0.2 to 0.9 wt.%.
Malachite according to claim 2, characterized in that the zinc compound contains malachite as basic zinc carbonate Zn 2 [CO 3] (OH) 2 or ZnCO 3 · Zn (OH) 2, and contain the impurity Fe 2 O 3, Na 2 O and residual ammonium ion into intercrystalline space.
-
Malachite according to any one of claims 1 - 3, characterized in that the malachite contains basic carbonic copper Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2, a zinc compound based on Zn +2 and impurities in following ratio, wt.%:
Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2 99,509-99,15
Zn +2 0,48-0,58
Fe 2 O 3 0.1-0.2
Na 2 O 0,01-0,1
Malachite according to any one of claims 1 - 4, characterized in that the microhardness of malachite 420-440 kg / mm 2.
Malachite according to any one of claims 1 - 5, characterized in that the wear resistance as compared with malachite natural wear malachite is 160-180%.
Malachite according to any one of claims 1 - 6, characterized in that the polishing malachite towards natural malachite polishability is 105-150% malachite density - 4.0-4.1 g / cm 3, hardness Mohs malachite - 4, and the maximum of the reflection spectrum of malachite - 500-535 nm.
Malachite according to any one of claims 1 - 7, characterized in that the contrasting contains malachite green alternating light and dark green layers and malachite surface in reflected light exhibits plush moire effect.
Malachite according to any one of claims 1 - 8, characterized in that the malachite is a polycrystalline aggregate obtained by evaporation of the solution of a basic copper carbonate and basic carbon dioxide in an aqueous solution of zinc ammonium carbonate.
A process for preparing malachite evaporation solution comprising basic copper carbonate in an aqueous solution of ammonium carbonate, characterized in that the basic solution evaporated copper carbonate and basic zinc carbonate in an aqueous solution of ammonium carbonate.
A method according to claim 10, characterized in that the basic solution evaporated carbonic copper Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2 and zinc basic carbonate Zn 2 [CO 3] (OH) 2 or ZnCO 3 · Zn (OH) 2 in an aqueous solution of ammonium carbonate at a ratio of zinc to copper in the solution was evaporated of from 0.018 to 0.09, preferably from 0.037 to 0.049 g per gram of Zn +2 Cu +2.
A method according to any one of claims 10 and 11, characterized in that the basic solution evaporated copper carbonate and basic zinc carbonate in aqueous ammonium carbonate solution is prepared by dissolving basic copper carbonate and basic zinc carbonate in an aqueous solution of ammonium carbonate with an excess ammonia in the molar content of 1 5-8 times relative to the molar content of carbon dioxide.
A method according to any one of claims 10 - 12, characterized in that the evaporation of the solution of a basic copper carbonate and basic zinc carbonate in the ammonium carbonate aqueous solution is carried out at a temperature of 40-95 ° C, preferably at a temperature of 60-80 ° C.
A method according to any one of claims 10 - 13, characterized in that the evaporation of the solution of a basic copper carbonate and basic zinc carbonate in the ammonium carbonate aqueous solution is carried out at a variable rate ensuring the possibility of crystallization of malachite, with alternating layers of contrasting, for example, light and dark green green.
A method according to claim 14, characterized in that the evaporation rate of the basic carbonic copper solution and the basic zinc carbonate in aqueous ammonium carbonate during the growth of the next layer of contrasting color with malachite shift change is not less than 1.2 times compared with the rate of crystallization during the evaporation previous layers of malachite.
A method according to any one of claims 10 - 15, characterized in that the evaporation of the solution of a basic copper carbonate and basic zinc carbonate in the ammonium carbonate aqueous solution is carried out by condensing the resultant solution by evaporation of the steam-gas mixture of NH 3, CO 2 and H 2 O to obtain an aqueous solution ammonium carbonate, which is used to dissolve the basic copper carbonate and basic zinc carbonate, and evaporation to obtain the feed solution of a basic copper carbonate and basic zinc carbonate in an aqueous solution of ammonium carbonate.
A method according to claim 16, characterized in that the evaporation of the solution and the basic copper carbonate basic zinc carbonate in the ammonium carbonate aqueous solution and condensing the resulting vapor mixture by evaporation of NH 3, CO 2 and H 2 O is carried out in a closed cycle.
A method according to claim 17, characterized in that the condensation of the resulting solution by evaporation of the basic copper carbonate and basic zinc carbonate in aqueous ammonium carbonate vapor-gas mixture of NH 3, CO 2 and H 2 O is carried out at a temperature of 30-55 ° C, preferably at a temperature 40-50 ° C.
A method according to any one of claims 10 - 18, characterized in that the evaporation of the solution of a basic copper carbonate and basic zinc carbonate in the ammonium carbonate aqueous solution is carried out until a predetermined thickness of malachite layer.
A method according to any one of claims 10 - 19, characterized in that the malachite prepared according to any of claims 1 - 8.
A process for preparing the malachite comprising evaporating solution of a basic carbonate of copper in an aqueous solution of ammonium carbonate, wherein the evaporating solution of a basic carbonate of copper in an aqueous solution of ammonium carbonate is carried out with condensation formed during evaporation NH gas mixture 3, CO 2 and H 2 O and produce an aqueous ammonium carbonate solution that is used to dissolve the basic copper carbonate and evaporated to obtain the feed solution of copper carbonate to basic aqueous solution of ammonium carbonate.
A method according to claim 21, characterized in that the evaporation of the solution in the basic copper carbonate aqueous solution, ammonium carbonate, condensing the resulting gas mixture by evaporation of NH 3, CO 2 and H 2 O, dissolving basic copper carbonate in an aqueous solution of ammonium carbonate and supplied to the receiving evaporating the solution of a basic copper carbonate in an aqueous solution of ammonium carbonate carried out in a closed cycle.
A method according to any one of claims 21 and 22, characterized in that the evaporation of the solution basic copper carbonate in an aqueous solution of ammonium carbonate is carried out at a temperature of 40-95 ° C, preferably at a temperature of 60-80 ° C, and evaporation of condensation formed during the vapor-gas mixture of NH 3, CO 2 and H 2 O is carried out at a temperature of 30-55 ° C, preferably at a temperature of 40-50 ° C.
A method according to any one of claims 21 - 23, characterized in that the evaporation of the solution of a basic copper carbonate in an aqueous solution of ammonium carbonate is performed to obtain a predetermined thickness malachite layer.
A method according to any one of claims 21 - 24, characterized in that the basic solution evaporated carbonic copper Cu 2 [CO 3] (OH) 2 or SuSO 3 · Cu (OH) 2 in an aqueous solution of ammonium carbonate with the addition of zinc carbonate basic Zn 2 [CO 3] (OH) 2 or ZnCO 3 · Zn (OH) 2 at a ratio of copper to zinc in the solution ranging from 0.018 to 0.09, preferably from 0.037 to 0.049 g per gram of Zn +2 +2 Cu, and the resulting by condensation gas mixture of NH 3, CO 2 and H 2 O aqueous ammonium carbonate solution is used to dissolve basic carbonic copper and basic carbonate of zinc to obtain a solution of a basic carbonate of copper and basic carbonate of zinc in aqueous ammonium carbonate in a closed loop to obtain malachite as claimed of claims 1 - 8.
print version
Publication date 02.12.2006gg



Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.