| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Technology market / Current models of the invention and / back / |
|
INVENTION
Russian Federation Patent RU2288301
Electrolyte for electropolishing SILVER
![]()
Applicant's Name: State Educational Institution of Higher Professional Education "Ivanovo State University of Chemistry and Technology" (VPO "ISUCT") (RU); Institute of Chemistry of the Russian Academy of Sciences solutions (ISC RAS) (RU)
Name of the inventor: Balmasov Anatoly Viktorovich (RU); Lilin Sergey (RU)
The name of the patentee: State educational institution of higher education "Ivanovo State University of Chemistry and Technology" (VPO "ISUCT") (RU); Institute of Chemistry of the Russian Academy of Sciences solutions (ISC RAS) (RU)
Address for correspondence:. 153460, Ivanovo, pr Engels 7, VPO "ISUCT" patent department
Starting date of the patent: 2005.05.04
The invention relates to the field of surface treatment processes silverware and can be used in mechanical engineering, instrument making and jewelery industry. Contains Electrolyte, g / l: 300-400 potassium thiocyanate or sodium, 20-40 glycerol, ethanol 1,7-6,7, 0,5-1,5 kaptaks, water to 1 liter. The technical result developed a nontoxic electrolyte allows to increase the resistance to tarnish the surface of products, reduce roughness and increase the reflectivity.
DESCRIPTION OF THE INVENTION
The invention relates to the field of surface treatment processes silverware and can be used in mechanical engineering, instrument making and jewelery industry.
Known cyanide silver electrolyte for electropolishing [Griliches SY Electrochemical and chemical polishing: Theory and Practice. The effect on the properties of metals. - 2 nd ed., Revised. and ext. L .: Engineering, Leningrad. Dep, 1987. 232 c], containing (g / l)..:

The disadvantages of analog are: the toxicity of a solution of electrolyte composition instability during polishing due to the difference of the anode and cathode current outputs, and the relatively high cost of the electrolyte due to the presence therein of silver cyanide.
Known cyanide silver electrolyte for electropolishing [Reference electroplating. Part 1: Trans. with it. / Ed. V.I.Laynera. M .: Metallurgy, 1969. 415 c], containing (g / l).:

The disadvantages of analog are: the toxicity of the solution, the high energy intensity of the process and the high cost of the electrolyte solution.
Known and ferrocyanide electrolyte polishing silver [Griliches SY Electrochemical and chemical polishing: Theory and Practice. The effect on the properties of metals. - 2 nd ed., Revised. and ext. L .: Engineering, Leningrad. Dep, 1987. 232 c], comprising the following components (g / l)..:

The disadvantages of this analogy is: the toxicity of the solution and the high energy intensity of the process.
The closest to the proposed electrolyte on a set of attributes, that is, the prototype is a thiocyanate aqueous electrolyte for the electropolishing of silver [T. - I.Yu.Yankauskas, P.A.Yuzikis, V.A.Kaykaris A.s.№497357 USSR. M. Cl. 2 to 23 in 3/06. Zayavl.22.06.73, Opubl.30.12.75, BI №48, 1976] containing (mol / l):
![]()
and electropolishing process mode:
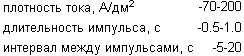
Disadvantages of the prototype are: poor resistance to tarnish the surface in an atmosphere containing sulfur compounds, the need to use high current densities and low reflectance surface due to the polishing process.
SUMMARY OF THE INVENTION
Inventive task was to develop a composition of a non-toxic water-organic electrolyte, which allows to increase the performance of silver electropolishing process, namely: 1) resistance to tarnishing, 2) leveling of the surface, manifesting itself in an increase in the relative height of the smoothing microroughnesses - ![]() Ra = (Ra beginning. -Ra Con.) / Rabeginning. and in reducing microroughness and 3) the reflectivity of the surface.
Ra = (Ra beginning. -Ra Con.) / Rabeginning. and in reducing microroughness and 3) the reflectivity of the surface.
The task is achieved by creating silver electropolishing electrolyte, comprising the following components (g / l):
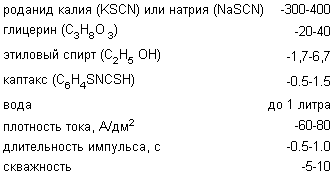
Comparative analysis of the prototype can be concluded that the inventive electrolyte differs from it in the introduction of new components, namely glycerol, ethanol and kaptaksa.
Sodium thiocyanate, GOST 10643-75, chemical formula NaSCN, melting point 287 ° C solubility in 166 g 100 g of water at 25 ° C and 225 g per 100 g water at a temperature of 100 ° C 100 ml water [Chemical Handbook, Volume .2, L .: Chemistry, 1964].
potassium thiocyanate, TU 264311-019-45225481-01 chemical formula KSCN, melting point 173.2 ° C, 217 g of a solubility in 100 g of water at 20 ° C and 670 g per 100 g water at a temperature of 100 ° C [Reference chemist tom.2, L .: Chemistry, 1964].
Glycerin, TU 264311-025-45225481-01, chemical formula C 3 H 8 O 3 density of 1.2641 g / cm 3, melting point 20 ° C., the solubility in 100 ml of water is equal to infinity [Reference chemist is. 2, L .: Chemistry, 1964].
Ethanol technical rectified, GOST 18300-87, chemical formula C 2 H 5 OH, density of 0.7813 g / cm 3, melting point -114,6 ° C, the solubility in 100 ml of water is equal to infinity [Reference chemist tom.2, L .: Chemistry 1964].
Kaptaks (2-mercaptobenzothiazole), GOST 739-74, chemical formula C 6 H 4 SMCSH, melting point 132 ° C, insoluble in water, sparingly in ether, dissolved in hot ethanol [Reference chemist tom.2, L. Chemistry, 1964].
DETAILED DESCRIPTION OF THE INVENTION
example 1
To prepare 1 liter of electrolyte to 400 g of potassium thiocyanate was dissolved in water at a temperature of 20-25 ° C, 40 g of glycerol was added as a solution in warm water, then added kaptaksa hot alcohol solution containing 4.2 g of ethanol and 1 g kaptaksa. Then the volume of the resulting solution was adjusted to 1 liter.
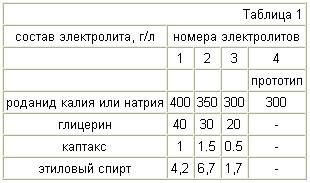
Examples of other values from the claimed concentrations of the electrolyte are shown in Table 1.
electropolishing process is carried out in a cell volume of 50 ml with stirring. The cathodes used copper plate area of 3 cm 2 and an anode of silver disk electrode area of 0.12 cm 2.
polishing mode
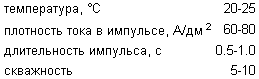
To increase the processing speed and improve the quality of the treated surface it is advisable to hold the silver electropolishing process in the pulsed mode at pulse widths ranging from 0.5-1.
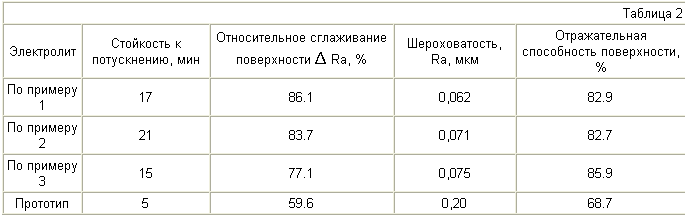
Surface roughness was measured quality characteristic (height of microroughness) and the reflectivity of the electrode surface before and after the polishing process. To determine the optimal concentration of glycerol in the solution of silver electropolishing investigated the dependence of surface roughness on the concentration of the organic component. The results are shown in Table 2.
Thus, seen from the table that administration of glycerol and ethanol in kaptaksa electrolyte for electropolishing silver allows to improve the surface state, namely, to increase resistance to tarnish in an atmosphere containing a sulfur compound (the time until the fogging increases in 3-4 times ) significantly improve the quality of the machined surface (the surface to increase the relative magnitude of smoothing 1.30-1.44 times and reduce the amount of 2,67-3,20 times roughness) and increase (1.25 times) the reflectance of the treated surface ( its luster).
Additional advantages of the electrolyte in comparison with the prototype are: significantly (2.2-fold) reduction in power consumption by reducing the operating current density from 70-200 to 60-80 A / dm2; increase the stability of the electrolyte - electrolyte performance is increased by 2 times (the proposed correction is performed after the passage of electrolyte therethrough 4.5 A h / l, while for the prototype this value is 2-2.5 A hour / liter).
CLAIM
Silver electropolishing electrolyte comprising potassium thiocyanate or sodium, characterized in that it additionally contains glycerin, and ethanol kaptaks the following component ratio, g / l:

print version
Publication date 02.01.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.