How much caffeine is contained in different drinks

Caffeine (also matein, guaranin) is a purine alkaloid, colorless or white bitter crystals. It is a psychostimulant, found in coffee, tea and many soft drinks. Caffeine is found in plants such as coffee tree, tea, cocoa, mate, guarana, cola, and some others. It is synthesized by plants to protect against insects that eat leaves, stems and grains, and to encourage pollinators.
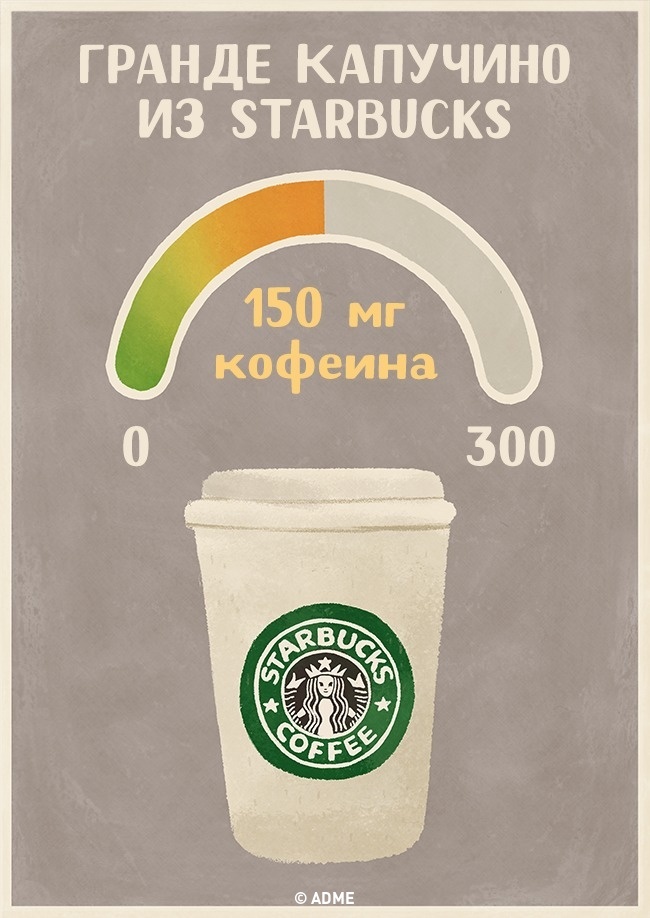
Knowledge of most people about caffeine is limited, for example: "There is a lot of coffee, in tea - less." This is fundamentally not true. In fact, there are unexpected nuances. We decided to see how much caffeine is contained in one standard portion of the drinks you know. And we also compared this amount with a safe daily intake of 300 mg of caffeine.
A cup of tea

A cup of green tea

Bank of Coca-Cola

A cup of black tea

Bank Mountain Dew

Espresso Cup

Red Bull Bank

A cup of instant coffee

A cup of ground coffee

Grande Cappuccino from StarBucks
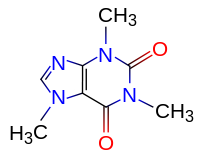
Are common:
- Systematic name: 1,3,7-Trimethyl-1H-purin-2,6 (3H, 7H) -dione
- Traditional names: 1,3,7-trimethylxanthine, guaranine, caffeine, matein, methylteobromine, tein
- Chem. Formula: C8H10N4O2
- Rat. Formula: C8H10N4O2
Physical properties:
- The state of the solid crystalline substance is colorless or white, odorless.
- Molar mass 194.19 g / mol
- Density 1.23 g / cm3
Thermal properties:
- M.f .: 234 ° C
- T. sub: 180 ° C
Chemical properties:
- PKa: 1.22
Structure:
- The dipole moment: 3.64 A
Classification:
- Reg. CAS number: 58-08-2
- PubChem: 2519
- Reg. EINECS number: 200-362-1
- RTECS: EV6475000
- ChemSpider: 2424
Security:
- LD50: 192 mg / kg (mice, orally)
- Toxicity: Moderately Toxic
In animals and humans, it stimulates the central nervous system, intensifies cardiac activity, speeds up the pulse, causes the blood vessels to expand (mainly the vessels of skeletal muscles, brain, heart, kidneys), increases urine output, reduces platelet aggregation (however, in some cases, opposite effects are noted) . This is due to the fact that caffeine blocks the enzyme phosphodiesterase, which destroys cAMP, which leads to its accumulation in cells. CAMP is a secondary mediator through which the effects of various physiologically active substances, primarily adrenaline, are realized. Thus, the accumulation of cAMP results in adrenaline-like effects.
In medicine, caffeine is used as a part of the remedies for headaches, migraines, as a stimulant for breathing and cardiac activity for colds, for increasing mental and physical performance, for eliminating drowsiness.
Dosages for caffeine should be selected individually. Higher doses for adults inside: single 0.3 g, daily 1.0 g, intravenously: single 0.4 g, daily 1 g.
Caffeine is on the list of vital and essential medicines.
History of the discovery
It was discovered and named "caffeine" in 1819 by the German chemist Ferdinand Runge. In 1827, Oudry isolated a new alkaloid from tea leaves and called it thein. Caffeine in its pure form was first obtained in 1828 (Pelletier and Cavant). In 1832, its composition was established by Weller and Pfaff with Liebig. In 1838, Iobst and G. Ya. Mulder proved the identity of theine and caffeine. The structure of caffeine was clarified by the end of the XIX century by Hermann Emil Fischer, who was also the first person who artificially synthesized caffeine. He won the Nobel Prize in Chemistry in 1902, which he received partly because of this work.
Chemical structure and properties
The chemical name of caffeine is 1,3,7-trimethyl-xanthine. In an alkaline medium (at pH> 9), it turns into caffeine C7H12N4O. By its structure and pharmacological properties, caffeine is close to theobromine and theophylline; All three alkaloids belong to the group of methylxanthines. Caffeine acts better on the central nervous system, and theophylline and theobromine - as stimulants of cardiac activity and mild diuretics. Caffeine, like other purine alkaloids, gives a positive murexide reaction, when heated with Nessler's reagent, caffeine forms a red-brown precipitate, unlike theobromine, giving under such conditions a light brown color.
Physical properties
White needles are bitter taste, odorless. It is soluble in chloroform, poorly soluble in cold water (1:60), easily - in hot (1: 2), it is difficult to be soluble in ethanol (1:50). Solutions have a neutral reaction; Sterilized at +100 C for 30 minutes. ??? m.p. 234 C.
Illustrator Natalya Roshchenko
Via http://www.caffeineinformer.com/the-caffeine-database


Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.